glomerular filtration
1/33
Earn XP
Description and Tags
week 4 ctb
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
urine formation
nephron= functional unit of kidney
3 main processes performed by nephron:
filtration
reabsorption
secretion
urinary excretion of any substance reflects sum of these processes
renal handling of substances
different substances handled in different ways by the kidney
processes can be altered according to needs of body to either lose or retain a substance
filtration is first step in process of urine formation
high rate of filtration needed to help clear substances from plasma efficiently
renal blood flow (RBF)
filtration requires good blood flow to kidney
kidneys receive 22% cardiac output (5l/min)
renal blood flow= 1100ml/min (haematocrti ~0.4)
renal plasma flow= 660ml/min
20% of renal plasma flow passes through filtration barrier to form filtrate
filtration fraction= CFR/RBF= 132ml/min
~180l/day of filtrate formed
renal corpuscle
renal corpuscle made up of glomerulus (glomerular capillaries) and Bowman’s capsule
arteriole at either end of glomerular capillary bed: facilitates high pressure in glomerulus for filtration
filters blood to form an initial ultrafiltrate
ultrafiltrate in Bowman’s capsule identical to plasma except it lacks plasma proteins and cellular components of blood
filtration barrier
glomerular filtration barrier formed from:
glomerular capillary endothelium (fenestrated)
basement membrane (-ve charge)
epithelial cells (podocytes) (interdigitating foot processes and filtration slits)
fenestrated endothelium
glomerular capillary endotheliu, is fenestrated
pores 70-100nM in diameter
water, solutes and plasma proteins can pass through pores
too small to allow cells and platelets through
basement membrane
similar but much thicker than other basement membranes (multi layered)
negatively charged
type IV collagen, laminin, fibronectin, entactin, other negatively charged glycoproteins
doesn’t permit filtration of plasma proteins
specialised epithelium
specialised epithelial cells called podocytes
primary processes wrap around the glomerular capillaries strengthen them against high pressures
secondary foot processes interdigitate to form filtration slits
foot processes are bound to basement membrane via nephrin to form a slit diaphragm
regulating filtration
limits passage of substances based on their size, charge and shape
blood cells and most plasma proteins (including substances bound to them) are excluded from filtrate
filtrate has an almost identical composition to plasma
disease processes may alter the properties of the barrier allowing protein to appear in the filtrate (proteinuria)
factors determining filtration
GFR= volume of filtrate formed by all the nephrons in both kidneys per unit time
determined by:
glomerular capillary filtration coefficient (Kf)
net filtration pressure (NFP)
GFR= Kf x NFP
filtration coefficient (Kf)
glomerular capillaru filtration coefficient, Kf reflects the:
surface area available for filtration
hydraulic conductivity (permeability) of the filtration barrier per unit area
changes in Kf aren’t the major part of the physiological regulation of GFR but may be affected in disease processes
eg reduced number of nephrons or processes which damage the filtration barrier will decrease SA or permeability, therefore decreasing GFR
net filtration pressure (NFP)
net filtration pressure is given by the sum of the pressures acting across the filtration barrier (Starling forces)
hydrostatic pressures (favours filtration: forces pushing water and other solutes out of the compartment they're in)
PG: glomerular (pushing plasma out of capillaries and into Bowman’s capsule)
PB: Bowman’s capsule (pushes filtrate
colloid osmotic (oncotic) pressures (opposing filtration: forces holding water/solutes in the compartment they’re in)
πG: glomerular
πB:Bowman’s capsule
typical NFP
oppose filtration:
Pb
Pig
favour filtration:
Pg
Pib
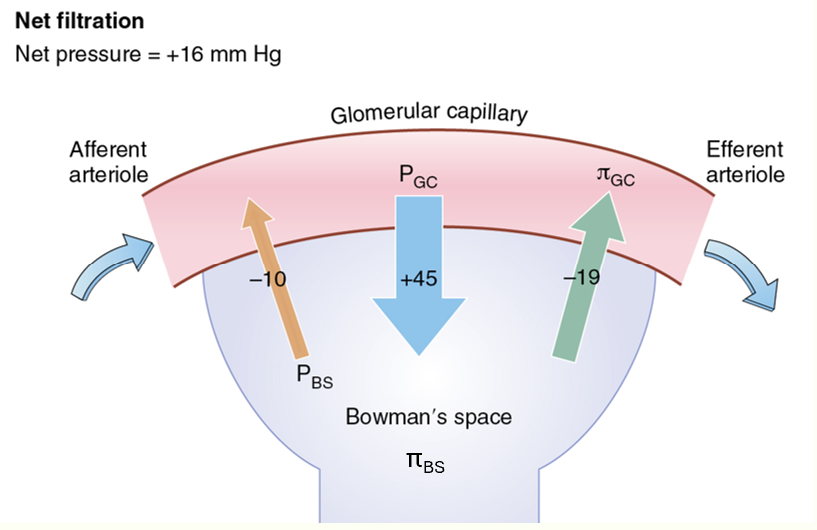
NFP= PG - PB - πG + πB
typical NFP diagram
NFP= 45-10-19+0
regulation of GFR
most physiological regulation of GFR occurs due to changes in glomerular hydrostatic pressure (PG)
PG depends on:
systemic arterial pressure
afferent arteriole resistance
efferent arteriole resistance
can vary PG by varying resistance of afferent and efferent arterioles
leaky hose analogy
can vary PG by varying resistance of afferent and efferent arterioles
balance of AA and EA resistances help determine GFR
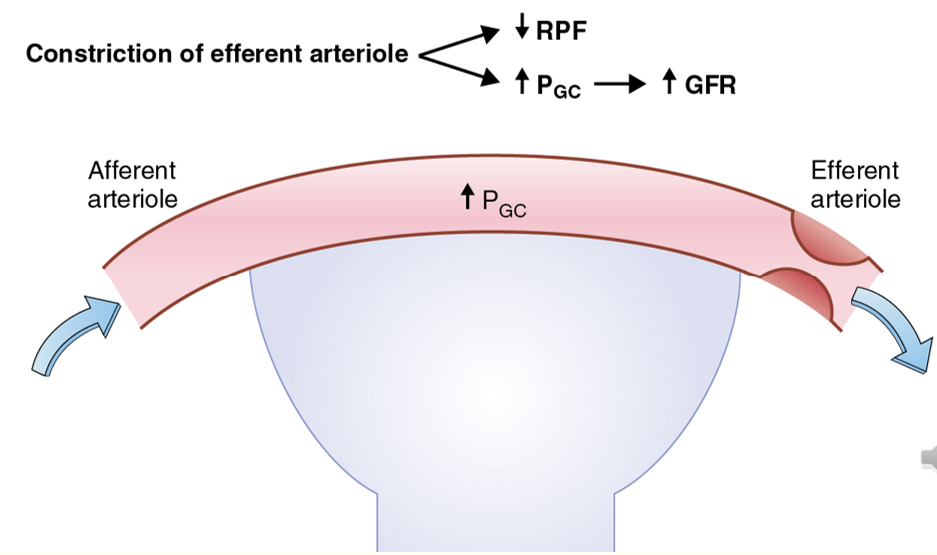
increasing GFR via PG
EA constriction increases GFR
AA dilation increases GFR
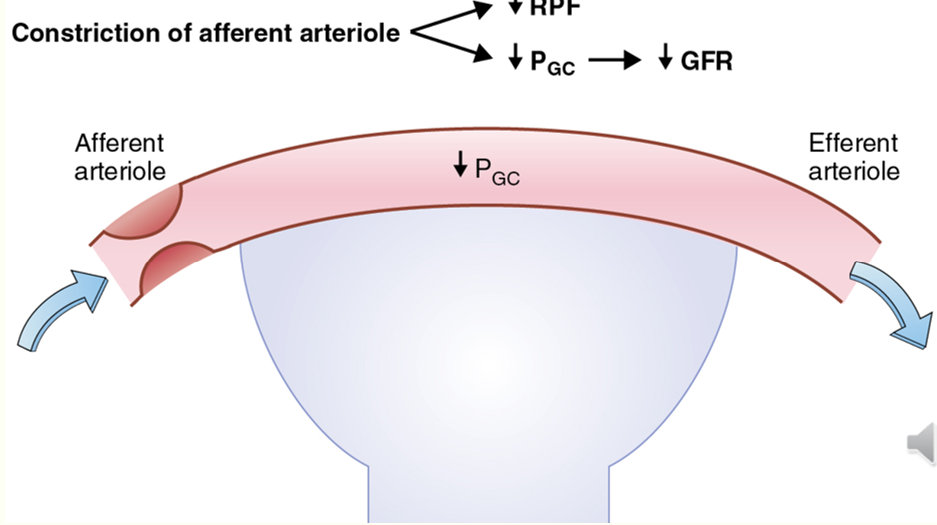
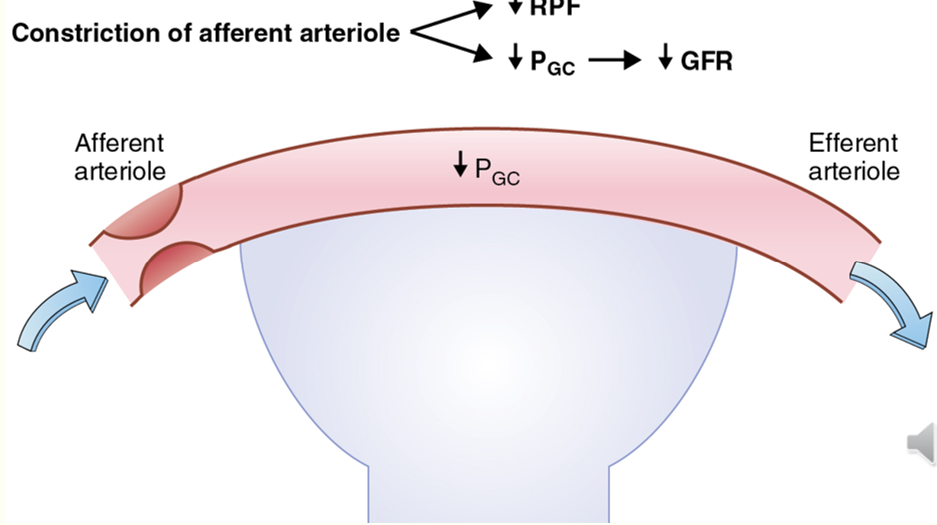
decreasing GFR via PG
AA constriction decreases GFR
EA dilation decreases GFR
balancing GFR
AA dilation and EA constriction increases GFR
AA constriction and EA dilation decreases GFR
humoral modulators of GFR
presenc/absence of vasoactive substances all have an effect on PG and therefore GFR
e.g:
angiotensin II preferentially constricts EA: therefore increasing PG
prostaglandins and artial natriuretic peptide (ANP) vasodilate AA: therefore increasing PG
noradrenaline, adrenosine and endothelin vasoconstrict AA: therefore reducing PG
autoregulation of GFR
renal blood flow and GFR stays relatively constant across a range of systemic blood pressures (80-180 mmHg)
prevents large fluctuations in renal excretion of water and soluted
2 main mechanisms of autoregulation
myogenic response
tubuloglomerular feedback
myogenic autoregulation
inherent ability of smooth muscle in afferent arterioles to respond to changed in vessel diameter by contracting/relaxing
increase in arterial BP
increased RBF and GFR
increased stretch of AA SMCs
opens Ca2+ channels
reflex contraction of AA smooth muscle
vasoconstriction of AA
increased resistance to flow
decreased RBD and decreased GFR
restoration of RBF and GFR to normal levels
tubuloglomerular feedback (TGF)
tubuloglomerular feedback mechanism links changes in NaCl in tubule lumen to constrol of own AA resistance in same nephron
utilises juxtaglomerular apparatus
juxtaglomerular apparatus
macula densa cells in early part of distal tubule sense (NaCl)
when arterial BP id increased, it causes increased GFR which increases flow and NaCl delivered to distal tubule
when arterial BP is reduced, it causes decreased GFR which decreases flow and NaCl delivered to distal tubule
TGF mechanism increases BP
Na+ and Cl- in filtered load sensed by NKCC2 transporter on macula densa cell membranes
paracrine mediator (adenosine) released by macula densa cells
AA vasoconstriction results in increased resistance and decreased PG
net result = decreased PG and restoration of GFR
TGF mechanism decreases BP
decreased release of paracrine factor from macula densa leads to AA dilation and decreased resistance
angiotensin II preferentially constricts EE
net result = increased PG and GFR
indicators of renal decline
urinanalyses and plasma analysis
indicated proteinuria/albuminuria
haematuria
indicate function
calcium/phosphate homeostasis
electrolytes/pH
fluid balance/urine vol
haemoglobin
indicates function
GFR/estimated GFR
serum creatinine/urea
measuring renal function (GFR)
GFR= vol of filtrate formed by all the nephrons in both kidneys per unit time
GFR is directly related to function of nephrons
declines in all forms of progressive kidney diseases
GFR= usually accepted as best overall index of kidney
linked to SA of body: typical young male GFR= 120 ml/min
GFR is linked to age, sex and body size: declines with increasing age
routine measures of GFR
if something that is normally entirely filtered by the kidney builds up in the blood it indicates decreased GFR and therefore decreased renal function
measurement using exogenous markers is more accurate but can be time-consuming and expensive (eg insulin infusion)
measurement via endogenous markers more cost effective and used routinely in clinical practice
3 tests used routinely to assess renal function
serum urea
serum creatinine
estimated GFR (eGFR)
they all use a single serum (blood) measurement and are therefore more convenient for the routine assessment and monitoring of renal function
serum creatinine
serum creatinine more accurately reflects GFR than urea
creatinine is formed from breakdown of creatine (skeletal muscle component)
usually produced at a steady rate for a given individual and is cleared from body fluid almost entirely by glomerular filtration
eGFR is relatively inaccurate as a point measure of GFR though useful for monitoring trends
inaccuracies result from way its production varies between individuals and relates to muscle mass
remains a useful clinical tool for monitoring trends in kidney function
estimated GFR (eGFR)
eGFR uses equations to calculate GFR based on a single serum measurement of a substance
incorporates simple clinical information along with single serum measurement to generate an eGFR
most use serum creatinine, age and sex to calculat
normal eGFR= >90ml/min
new tests use serum cystatin C (eGFRcystatinC)
limitations of eGFR
no part of CKD-EPI equation includes a measure of body size and is based on serum creatinine, age and sex
eGFR results can be influenced by factors that alter muscle mass
limitations on its use
children
AKI
drug dose calculations for highly toxic drugs
eGFR more accurate than serum creatinine alone
useful in monitoring renal function and in detecting early decline