L6 Reduction of C-C multiple bonds
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
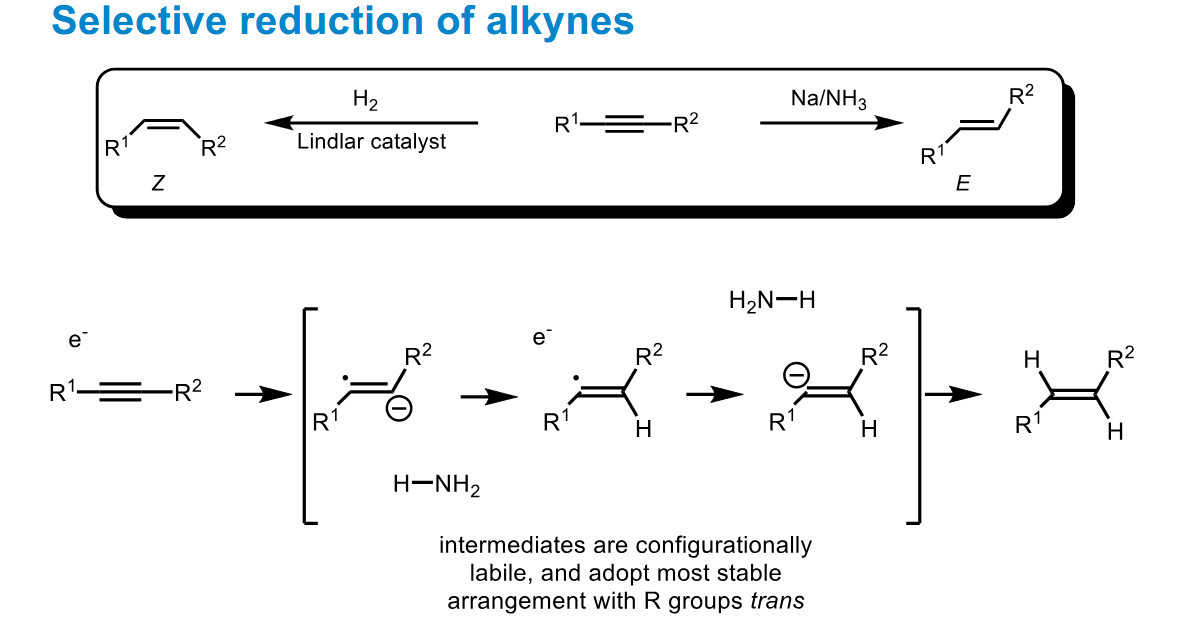
Reduction of alkynes to alkenes - 2 methods
What is the stereoselectivity of each
Note how the catalyst favours Z - adsorption and the dissolving metal reduction favours E due to sterics.
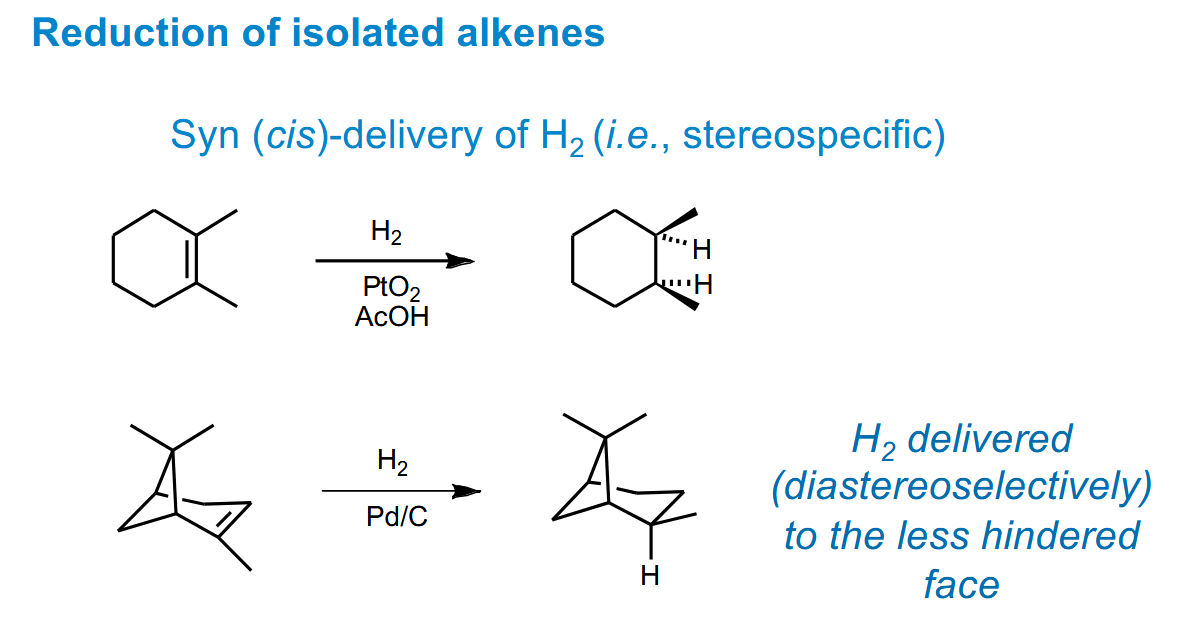
Stereospecific/selective control of reduction of isolated alkenes
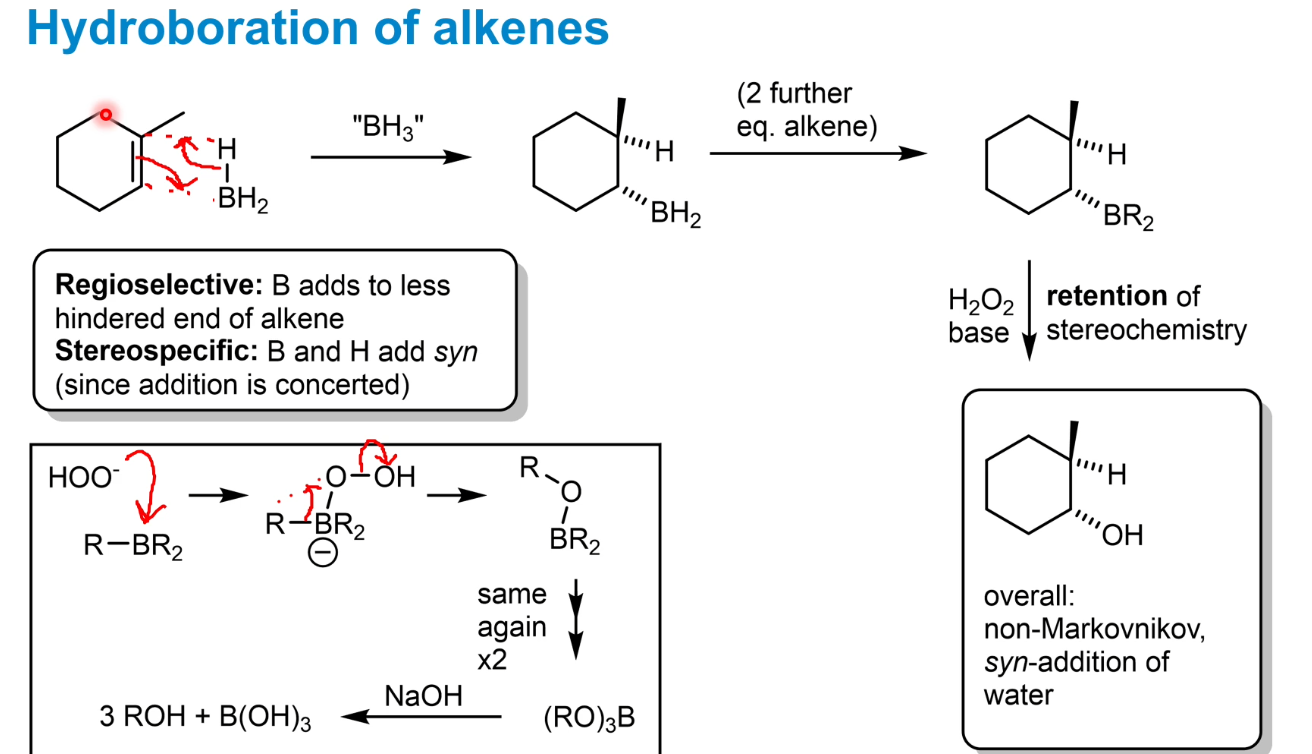
Hydroboration of alkenes selectivity features
Regioselective - B adds to less hindered end - producing the anti-Markovnikov product
Stereospecific - always syn- addition as concerted reaction
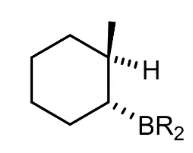
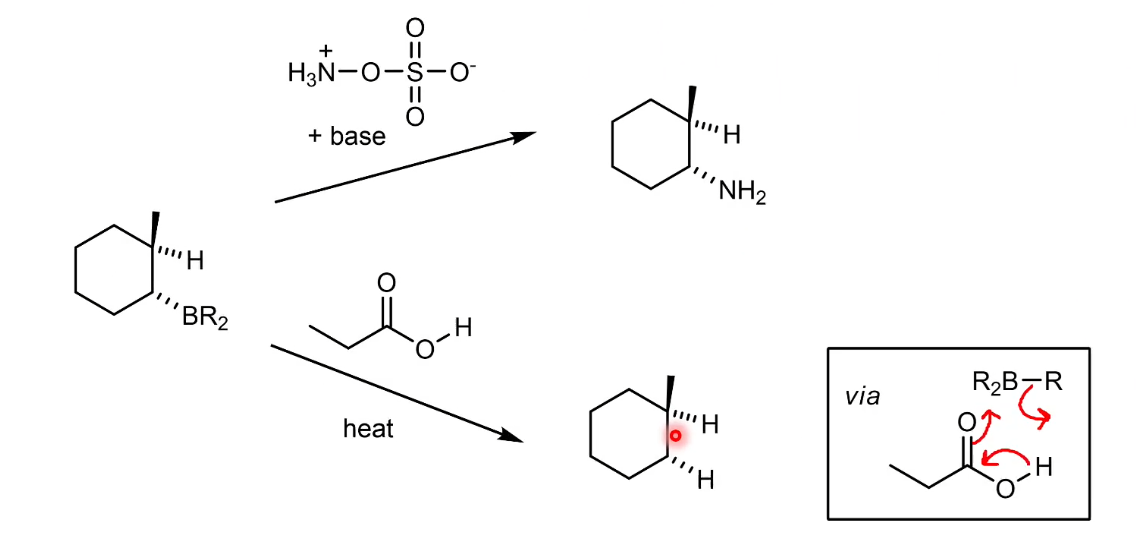
What are other things you can do with the boron structure (add H or NH2
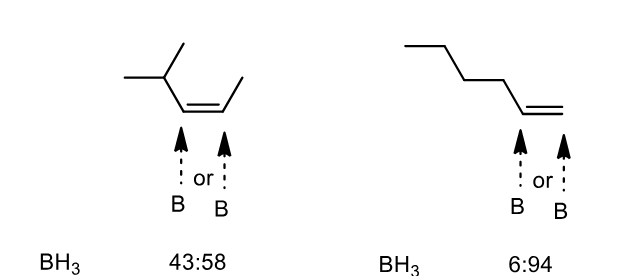
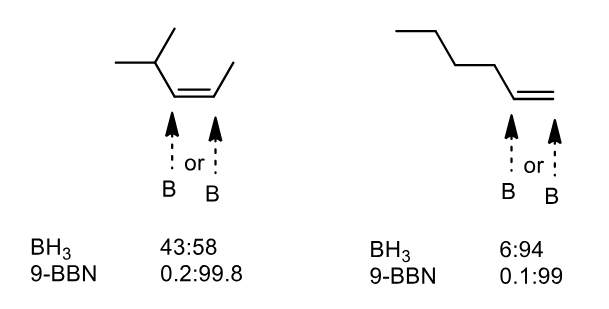
Some alkene structures have similar sized R groups and hence the regioselectivity is poor. How can we improve it
By making very hindered reagents. Notice the increase in selectivity is huge.
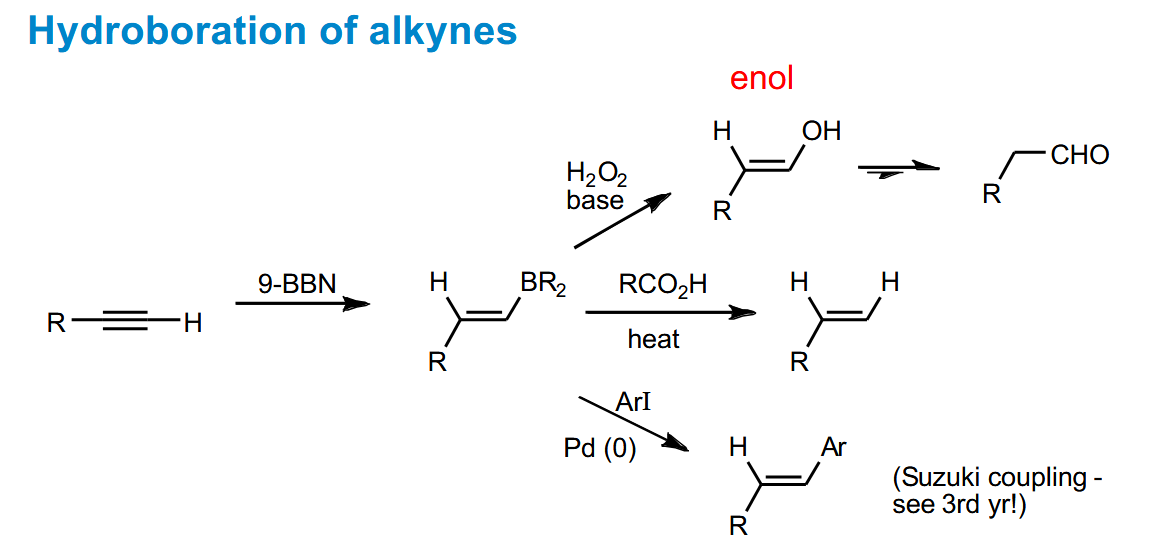
Hydroboration of alkynes to form carbonyls, terminal alkenes and aromatic alkenes
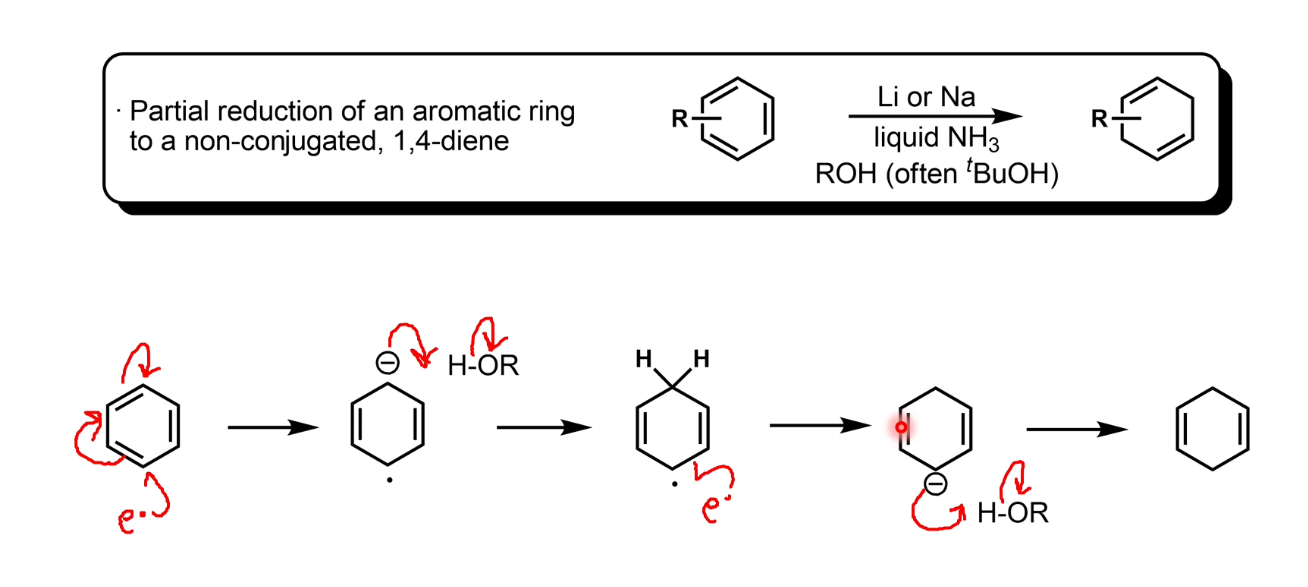
Partial reduction of unsubstituted aromatics: Birch reduction
Note the lack of conjugation (makes it less stable) - this is the kinetic product
Intermediate is not basic enough to deprotonate NH3 so an alcohol is added to provide protons
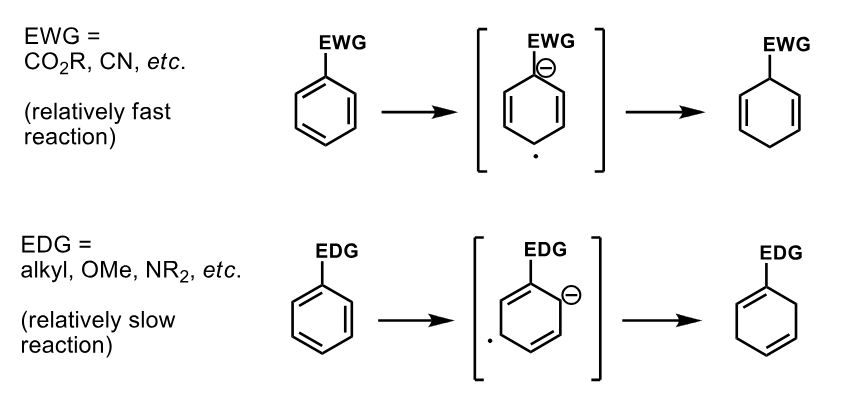
How does the presence of an EDG / EWG affect the rate of the Birch reduction
EWG - fast as it lowers the LUMO energy
EDG - slow as it raises energy of LUMO pi*
Also moves the position of the -ve charge away form the EDG
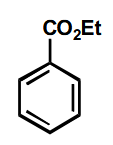
Adding an electrophile to the same carbon
The EWG forces the negative charge onto this carbon.
The charge ends up next to the group as the negative charge at the para- position moves as the proton is more acidic on carbon 1 so intramolecular proton transfer occurs.
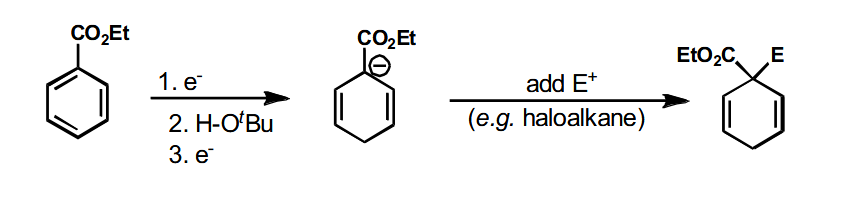
Using 2 FGs to direct the charge
Both FGs direct the negative charge to the same spot. This reaction has no added alcohol as the proton comes from the carboxylic acid. The final product is formed via a multitude of steps.
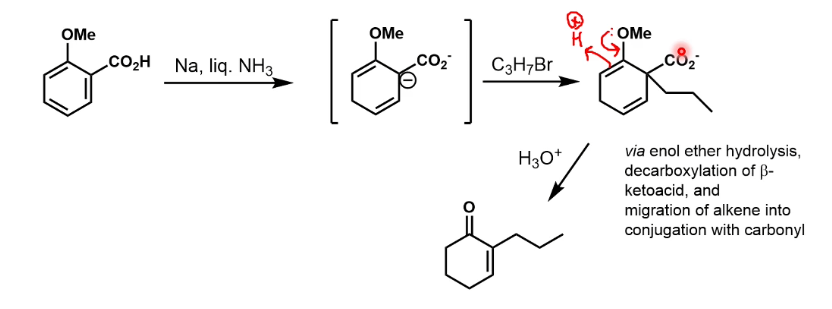
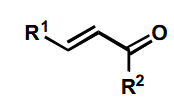
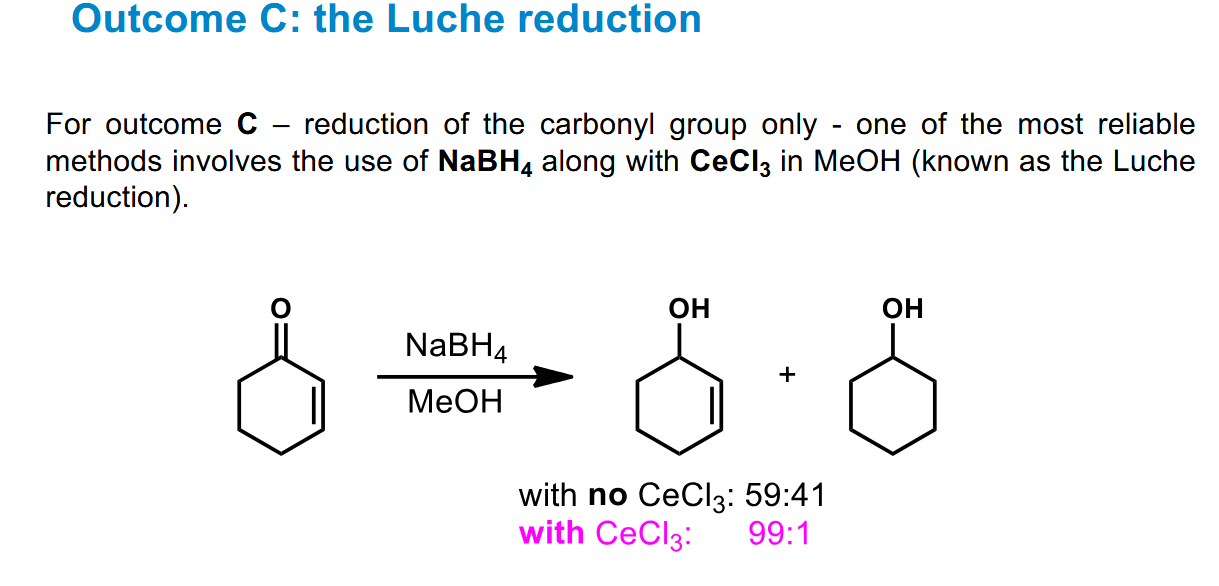
Possible outcomes of reduction of this
First one is easily done by LiAlH4 or catalytic hydrogenation.
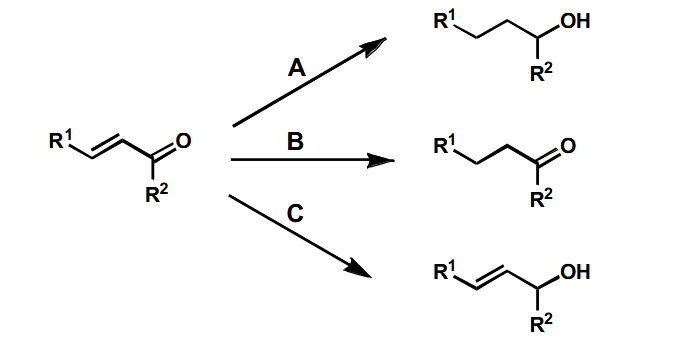
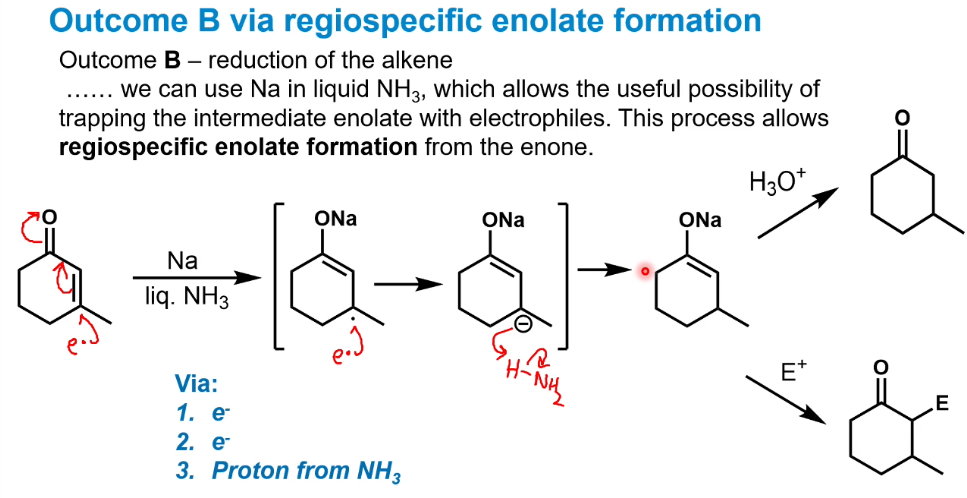
Selective reduction of the alkene
Using dissolving metal reduction.
Selective reduction of the carbonyl - Luche reduction
NaBH4 and an alcohol produces a fairly even mix of 1,2 and 1,4 addition - however the addition of CeCl3 pushes the major product massively in the direction of the 1,2 addition, leaving the alkene intact