Chem 11 Unit 8: Atomic theory (2-1)
1/27
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Properties of Metals (5)
Solid at room temp (Except Hg)
Shiny/lustrous when freshly cut/polished
Good conductors of heat + energy
Generally malleable & ductile
Forms cations
Properties of Metalloids (5)
When temp increases
Metal → conductivity decreases
Metalloids → conductivity increases
B, Si, Ge, As, Sb, + Te = confirmed metalloids
Po + As = debateable
Po = metal OR metalloid
As = Non-metal OR metalloid
Properties of Non-metals (4)
Usually gases/brittle solids at room temp (except liquid bromine)
Bad conductors of heat and energy
Solids are dull to lustrous in appearance and opaque to translucent
Forms anions
Periodic table trend principles (3)
e- in large orbits = farther from the nucleus, less attraction to p+ bc more shielding
More p+ = more attraction for e- from nucleus→ effective nuclear charge (Zₑff)
e- in the same subshell repel each other
Atomic Radius (3)
Measure of the radius from the nucleus to the Ve- in an atom
Down group → AR increases: More shells (higher n value) + shielding
Across period → AR decreases: Zeff & Protons increases (Added e- joins same orbital)
Ionic Radius (4)
AR of an ion
Cations = smaller than neutral
Loses electrons so AR decreases
Anions = larger than neutral
Gains elections so AR increases
Ionization Energy (IE) (6)
Amount of energy needed to remove an e-
First ionization energy (IE₁): the energy required to remove the first e-
Trend = opposite to AR, small AR → high IE
Elements on left need to get rid of e- to become stable
Elements on right need to gain e- to become stable
Exceptions: O = lower IE₁ than N bc O wants to lose 1e- to become half filled
Electronegativity (EN) (5)
The ability to attract e-
Measurement of an atom’s Zeff on OTHER atoms
Atoms w/ high EN pull bonded e- closer to their nuclei
Noble gasses have no EN value (shells stable)
EN has the same trend as IE
Metallic Character (2)
Down group = more metallic
Across period = less metallic
Intramolecular interactions (2)
Occur WITHIN molecules/compounds/crystals
Includes: ionic, covalent, metallic bonds
Ionic Bonds (5)
Forms when large difference between EN and IE of both atoms (EN >1.8)
Metal w/ low IE/EN transfers Ve- → Non-metal w/ high IE/EN
Electrostatic attraction-both atoms become ions after transfer
Sometimes forms into a crystal lattice (Ex. NaCl)
Makes compound have a high melting point (Caused by a vast number of attractive forces in structure)
Covalent bonds (gen) (3)
Non-metal and non-metal
Share valence electrons
Tend to have lower MP than ionic compounds
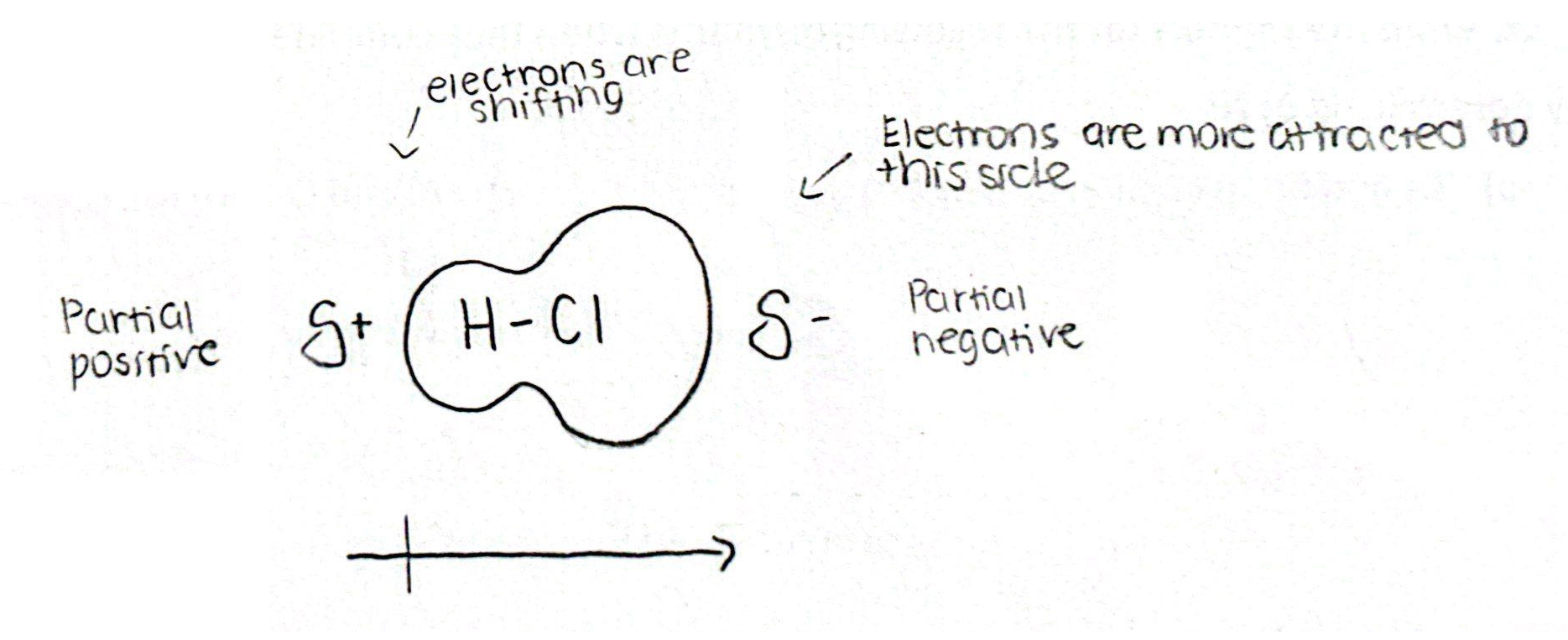
Polar Covalent Bonds (3)
Forms when EN = 0.5-1.8
When e- are pulled more towards one atom bc of higher EN, one side of the moc becomes more negative than the other side (unequal sharing of e-)
Causes dipole (One side more negative)
Non-polar Covalent Bonds (7)
Forms when EN < 0.4
Between identical/same charged non-metals
EN value = same; ΔEN = 0
Collision of atoms = electron clouds overlap
Attractive forces (nucleus) may begint o overlap repulsive forces (e-)
Pair of Ve- shared between both
The electrons will not be more attracted to one nuclei over the other (equidistant)
Metallic Bonds (3)
Protons swarmed by electrons
“sea” of electrons makes metal malleable + conductive
Forms metallic crystals
Early Atomic Theory Timeline (6 ppl)
Democritus (he was right all along)
First coherent atomic theory
Matter is made of tiny indivisible particles called atoms
Aristotle (More influential)
Everything is made up of 4 elements: Fire, air, earth, water
Criticized Democritus because it didn’t explain properties of matter or chemical reactions
John Dalton
First “modern” atomic theory (Revived Democritus’ theory through experiments)
Atoms = solid spheres (different types of spheres make up different elements)
Proposed Billiard Ball Model
Spheres arranged in a triangle
J.J. Thomson
Discovered electron (Atoms are not the smallest particles of matter - there are more inside)
Aluminum Plates in a Cathode Ray Tube
Proposed Plum Pudding Model
Positive sphere w/ negative charged particles
Ernest Rutherford
Discovered nucleus (proton confirmed + neutron proposed)
Gold Foil Experiment: A lot of α particles went through, some rebounded. Like a net.
Proposed Planetary/nuclear Model
Positive charge in center/nucleus w/ electron surrounding it
Flaw: What is stopping the e- from meeting the p+? Atoms would just collapse.
Niels Bohr
Positive nucleus w/ electrons orbiting in shells of different energy levels (higher shell = higher energy)
Chadwick proposed neutrons
Periodic Table history (4 ppl - not important?)
William Odling:
Proposed elements could be divided into 13 groups based on chemical & physical properties
John Newlands:
Assigned H a mass of 1
Law of Octaves: Every 8th element shares similar properties (only up to Ca)
Did not allow for prediction of new elements. Table had to be rearranged each time a new element was discovered.
Dimitri Mendeleev:
Organized elements by atomic mass and properties.
Recognized periodic recurrence of properties, leaving gaps for undiscovered elements.
His (first widely recognized) table helped chemists organize data and predict new properties.
Periodic Law: Elements ordered by increasing atomic mass (later revised to atomic number) exhibit similar recurring properties.
Henry Moseley:
Proposed atomic number - revised periodic law slightly
Elements still grouped by properties, similar properties in the same column
Added a column of elements Mendeleev didn’t know about
Modern table:
Strutt & Dorn added noble gases in 1894
Lanthanides & Actinides added mid 1900’s
Continuously changing, currently at 118 elements
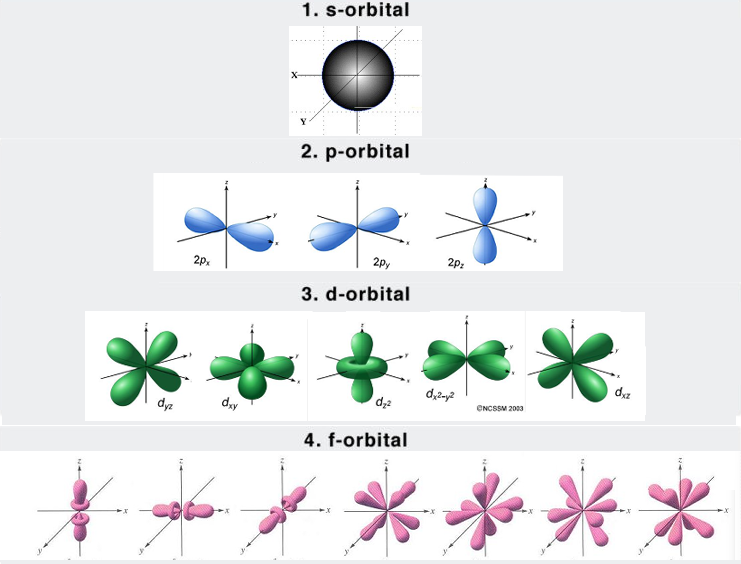
Energy Levels & Orbitals (4)
Energy difference betw 2 levels is called the QUANTUM energy
Electrons occupy regions of space called orbitals in a particular energy level (THEY DON’T ORBIT)
SHELL: The set of orbitals having the same n value
Ex. 3rd shell = 3s, 3p, 3d
SUBSHELL: A set of orbitals of the same type
There’s a set of five 3d orbitals in the 3rd subshell
Core Notation/Exceptions (4)
Anions: Add an electron to the last unfilled subshell
Cations: Remove an electron from the highest energy subshell
Ex. 4s before 3d
Ex. np e- before ns e- before nd e-
Exceptions: Cr & Cu - d subshell wants to be half or fully filled (most subshells do, but esp d)
Valence: all e- except core e- and filled d or f subshells. Noble gases and isoelectronic atoms/ions have 0 valence.
Orbital rules (3)
Aufbau Principle: Electrons fill the LOWEST energy orbitals first.
Pauli Exclusion Principle: Max of 2 electrons per orbital
Hund’s Rule: Each orbital must have a single electron before doubling up with an e- of opposite spin
Isotopes & Percent abundance (3)
Isotopes: Different form of same element - Same # protons, diff # neutrons
Mass # is always WHOLE (Proton + Neutron)
Atomic mass = weighted avg of all existing isotopes
Emission Spectra (7)
When light hits electrons, they jump up a shell
If it isn’t the right coloured light, it won’t move
Ex. e- that needs red light won’t move if it’s hit with a yellow light
When they drop down a shell they release specifically coloured light
Proves the existence of e- shells
Solid lines of colour are observed
If there was no shells, it’d be a blur of colour
Families / Groups (10)
G1: Alkali Metals
Very reactive
Reactivity decreases down group
Forms 1+ ions
G2: Alkali Earth Metals
Reactive but not as much as Alkali Metals
Reactivity decreases down group
Forms 2+ ions
G3-12: Transition Metals
D-orbitals filled last (except Cr, Cu, + Au)
Forms many ions (multivalent)
G13: Boron Group
Forms 3+ ions
G14: Carbon Group
Forms 4+ ions (C can form 4- ions)
G15: Nitrogen Group
Forms 3- ions
G16: Oxygen Groups
Forms 2- ions
G17: Halogens
Very reactive
Reactivity increases up group
G18: Noble Gases
Very low reactivity, very stable, full Ve- shell
Hydrogen
Shares properties w/ many families
Octet Rule (5)
All atoms form stable octet when bonding
Stable + low-energy config.
EXCEPTIONS (sometimes):
H makes 1 bond
B takes less
S and P take more
Intermolecular Forces (4)
Occurs BETWEEN covalent molecules
Strength/weakness affects state of matter + chemical properties
Strong IMF = High BP (solid/liquid)
Weak IMF = Low BP (liquid/gas)
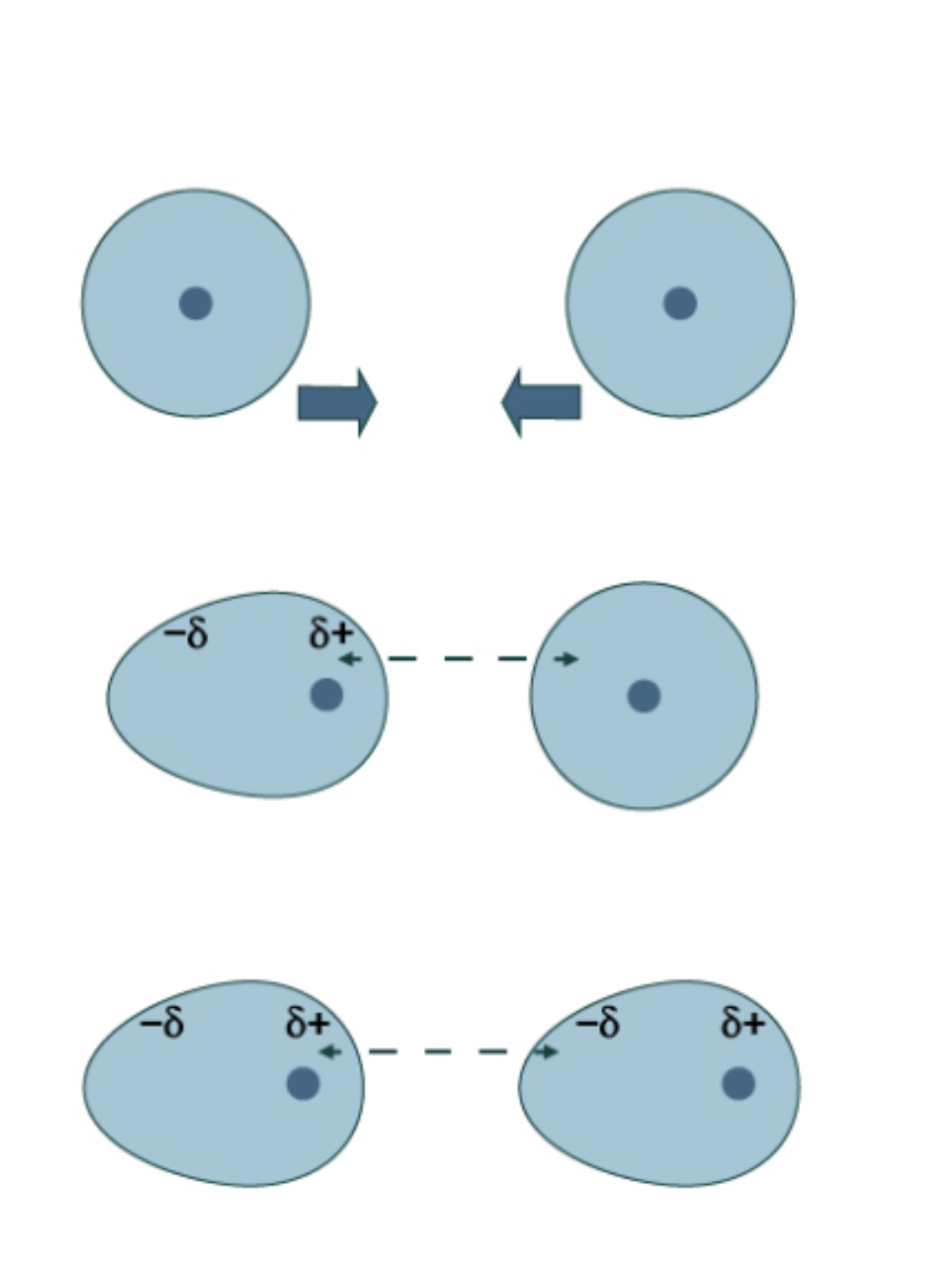
London Dispersion Forces (11)
Weakest force
Between 2 non-polar molecules
Always present
Creates temporary dipole
E- from molec1 → nucleus of molec2
Molec2 e- repulsed by molec1 e-
Attraction increases w/ atomic number
Dipole increases when more e- is added
Attraction increases w/ larger molecules
More spaces to attract
If only LDF present → low BP + MP
Dipole-dipole (4)
2nd strongest force
Between 2 polar molecules
δ+ from molec1 attracted to δ- from molec2
Stronger bonding = Higher BP + MP
Hydrogen Bonding (5)
Strongest force
Type of dipole-dipole (only for H)
H bonded with atoms with high EN
Ex. N, O, F
Molecules share a H