3.1 - Chemical Measurements
1/7
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
What is the law of conservation of mass?
States that no atoms are lost or made during a chemical reaction so the mass of the products equals the mass of the reactants.
Write a balanced equation of magnesium reacting with hydrochloric acid.
Mg(s) + 2HCl(aq) → MgCl2 (aq) + H2 (g)
Define relative atomic mass.
Average mass of atoms in an element taking into account masses and abundance of its isotope, relative to 12C.
Define relative formula mass.
Sum of RAM’s of all atoms in the formula.
What is the relative formula mass of CaF2?
CaF2 - (Ar values: Ca = 40 || F = 19)
40 + 19 + 19 = 78
What is the relative formula mass of C6H12O6?
C6H12O6 - (Ar values: C = 12 || H = 1 || O = 16)
(12 × 6) + (1 × 12) + (16 × 6) = 180
The following reaction occurs in a test tube under a Bunsen burner:
4MgO(s) + CH4 (g) → 4Mg(s) + 2H2O(g) + CO2 (g)
Using the equation, explain why the carbon dioxide and water escape from the test tube.
They are both gases.
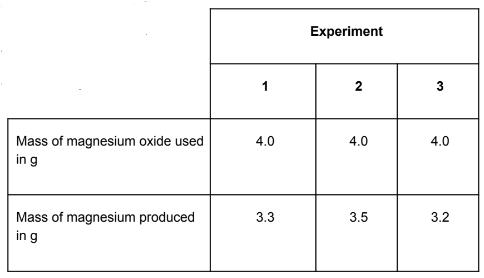
The experiment was repeated three times. Calculate the mean mass of magnesium produced and suggest how you could increase the precision of the results.
(3.3 + 3.5 + 3.2) ÷ 3 = 3.3
Measure to more decimal places OR use a more sensitive balance / apparatus.