ALL BIOLOGY SL 2025 EXAMS
1/1037
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
1038 Terms
Define cohesion
Cohesion is the attraction between molecules of the same substance, such as the hydrogen bonds between water molecules.
Describe how water moves through the xylem of a vascular plant
Water moves through the xylem by a combination of cohesion, adhesion, and transpiration. Cohesion helps water molecules stick together, while adhesion allows them to stick to the walls of the xylem, and transpiration creates a pulling force.
Outline the cause of surface tension
Surface tension is caused by the cohesive forces between water molecules at the surface, where they form stronger hydrogen bonds since there are fewer adjacent molecules to bond with.
State a benefit to living things that results from surface tension
Surface tension allows small organisms, such as insects, to walk on water without sinking.
Define adhesion
Adhesion is the attraction between water molecules and other substances that are polar or charged.
Define polar
Polar refers to a molecule that has regions of partial positive and partial negative charges due to unequal sharing of electrons.
Define ion
An ion is an atom or molecule that has gained or lost one or more electrons, resulting in a positive or negative charge.
Compare cation and anion
A cation is a positively charged ion, while an anion is a negatively charged ion.
Explain why water is attracted to molecules that are polar or charged
Water is attracted to polar or charged molecules because the partial charges in water can interact with the charges of other polar or ionic substances, leading to electrostatic attraction.
Outline the cause of capillary action
Capillary action occurs due to the combination of cohesion between water molecules and adhesion between water and the walls of a narrow tube, causing water to rise, e.g. during transpiration.
Describe capillary action in plant tissue
In plant tissue, capillary action allows water to move up the small xylem vessels, helping to transport water from the roots to the leaves.
Outline the cause and effect of capillary action in soil
Capillary action in soil is caused by the adhesion of water to soil particles and cohesion between water molecules, which helps water move through small spaces between soil particles, affecting moisture availability for plants.
Identify solvent and solutes of a solution
The solvent is the substance that dissolves the solutes, while solutes are the substances being dissolved in the solvent.
Define solvation
Solvation is the process of surrounding solute particles with solvent molecules to form a solution.
Explain why water is able to dissolve charged and polar molecules
Water dissolves charged and polar molecules due to its polarity, allowing it to form hydrogen bonds or electrostatic interactions with solutes.
Outline the solvation of hydrophilic and hydrophobic substances
Hydrophilic substances dissolve easily in water due to their polar or charged nature, while hydrophobic substances do not dissolve in water due to their non polar nature
State an example of the function of a molecule depending on it being hydrophobic and insoluble
Lipids function as a barrier in cell membranes due to their hydrophobic and insoluble nature, preventing water from passing through.
State an example of the function of a molecule depending on it being hydrophilic and soluble
Glucose is hydrophilic and soluble in water, allowing it to be transported in the bloodstream and used as an energy source.
Outline the role of water as a medium for metabolism
Water acts as a medium for metabolism by dissolving reactants and products, facilitating biochemical reactions in cells.
Describe the role of water as a medium for transport in vascular plants
Water carries nutrients and minerals through the xylem from the roots to the leaves, supporting plant growth and photosynthesis.
Describe the role of water as a medium for transport in animal blood
Water in blood acts as a medium for transporting oxygen, nutrients, waste products, and hormones throughout the body.
Define physical property
A physical property is a characteristic of a substance that can be observed or measured without changing its chemical composition.
List physical properties of water that are consequential for animals in aquatic habitats
Water's high surface tension, buoyancy, viscosity, high specific heat capacity, and thermal conductivity are important for animals in aquatic habitats.
Outline the cause and effect of buoyancy
Buoyancy is caused by the upward force exerted by water on objects, allowing animals to float or reduce their body weight in water.
Outline the cause and effect of viscosity
Viscosity is caused by the internal friction between water molecules, affecting how easily animals can move through water.
Compare viscosity of air to water to blood
Air has the lowest viscosity, water has a higher viscosity, and blood has the highest viscosity due to the presence of cells and proteins.
Define thermal conductivity
Thermal conductivity is the ability of a substance to transfer heat.
Compare less conductive to more conductive materials
Less conductive materials transfer heat slowly, while more conductive materials transfer heat quickly.
Outline a consequence to life of the thermal conductivity of air and water
Water's higher thermal conductivity compared to air allows aquatic animals to lose heat more quickly, which affects their body temperature regulation.
Define specific heat capacity
Specific heat capacity is the amount of heat required to raise the temperature of a substance by a given amount.
Describe why water has a high specific heat capacity
Water has a high specific heat capacity due to the hydrogen bonds between water molecules, which require more energy to break.
State two benefits to life of the high specific heat capacity of water
It stabilizes aquatic environments by preventing rapid temperature changes and helps regulate body temperature in organisms.
Outline a benefit to life of water's high specific heat capacity
Water's high specific heat capacity allows aquatic habitats to remain stable, providing a consistent environment for living organisms.
Compare the physical properties of water to those of air
Water has higher viscosity, higher specific heat capacity, and higher thermal conductivity compared to air.
Describe how the black-throated loon (Gavia arctica) and/or the ringed seal (Pusa hispida) interact with the physical properties of water in their habitat
Black-Throated Loon (Gavia arctica):
Adapted to water's density and buoyancy by having dense bones that reduce buoyancy, allowing it to dive efficiently for fish.
Streamlined body and webbed feet help minimize resistance in water, compensating for its higher viscosity.
Uses thermal stability of water to maintain body temperature while diving and feeding in cold waters.
Ringed Seal (Pusa hispida):
Exploits water's density and buoyancy to float effortlessly and swim efficiently while searching for prey beneath the ice.
Thick layer of blubber provides insulation against the cold, leveraging water's thermal conductivity to conserve heat in frigid environments.
Seals utilize water's high oxygen content by surfacing to breathe but can remain underwater for extended periods due to efficient oxygen use, compensating for the low oxygen availability in water.
List reasons why water is a substance on which life depends
Water is a universal solvent, helps regulate temperature, is involved in metabolic processes, facilitates nutrient transport, and provides a medium for chemical reactions essential for life.
Describe the structure of an atom
An atom consists of a nucleus containing protons and neutrons, with electrons orbiting the nucleus in energy levels or shells.
Outline the formation of ionic and covalent bonds between atoms
Ionic bonds form when electrons are transferred from one atom to another, resulting in oppositely charged ions. Covalent bonds form when atoms share pairs of electrons.
Explain the sharing of electrons between atoms in a polar covalent bond
In a polar covalent bond, electrons are shared unequally between atoms, leading to a slight charge difference across the molecule due to differences in electronegativity.
State the location of the polar covalent bond within a water molecule
The polar covalent bonds in a water molecule are found between the oxygen atom and each of the two hydrogen atoms.
Explain the partial charges of the oxygen and hydrogen atoms within a water molecule
In a water molecule, the oxygen atom has a partial negative charge (δ-), and the hydrogen atoms have partial positive charges (δ+) due to the unequal sharing of electrons.
Draw a water molecule, including notation to depict the partial charges of the atoms
H₂O structure shows one oxygen atom bonded to two hydrogen atoms with polar covalent bonds. Oxygen has a partial negative charge (δ-), and each hydrogen has a partial positive charge (δ+).
Outline the cause of the formation of hydrogen bonds between water molecules
Hydrogen bonds form due to the attraction between the partial negative charge (δ-) on the oxygen of one water molecule and the partial positive charge (δ+) on the hydrogen of another water molecule.
Outline the consequences of the collective strength of hydrogen bonds between water molecules
The collective strength of hydrogen bonds gives water unique properties such as high surface tension, high specific heat capacity, cohesion, adhesion, and the ability to act as a solvent.
Outline the role of complementary base pairing in maintaining the DNA sequence during DNA replication
Complementary base pairing ensures that each original DNA strand serves as a template for a new strand, with adenine pairing with thymine and cytosine pairing with guanine. This pairing mechanism allows accurate copying of the genetic code during DNA replication.
Outline the role of complementary base pairing in transmitting the genetic code in transcription and translation
In transcription, complementary base pairing ensures that RNA is synthesized accurately from the DNA template. In translation, the complementary base pairing of tRNA anticodons with mRNA codons ensures that the correct amino acids are added to the growing polypeptide chain, translating the genetic code into a protein.
Outline why there is a limitless diversity of DNA base sequences
The diversity arises from the four different nitrogenous bases (adenine, thymine, cytosine, and guanine) and their varying sequences and combinations. This allows for an immense number of possible sequences and combinations, providing a vast capacity for storing genetic information.
Define universal in relation to the genetic code
Universal means that the genetic code is nearly identical across all living organisms, indicating a shared evolutionary origin.
Outline why conservation of the genetic code across all forms of life is evidence of common ancestry
The conservation of the genetic code suggests that all life shares a common ancestor from which the genetic code was inherited. The similarity in the code across diverse organisms supports the idea of a universal common ancestry.
State the two primary functions of nucleic acids
Nucleic acids store genetic information and enable the synthesis of proteins.
State the two types of nucleic acids used in cells
The two types of nucleic acids are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
Outline the meaning and implication of DNA being the genetic material of all living organisms
DNA carries the genetic instructions for the development, functioning, growth, and reproduction of all known living organisms, implying a common evolutionary origin.
State why RNA viruses do not falsify the claim that all living things use DNA as the genetic material
RNA viruses are not considered living organisms, as they require a host cell to replicate, thus they do not contradict the claim that all living organisms use DNA as their genetic material.
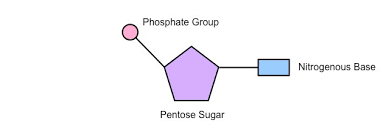
List the three components of a nucleotide
A nucleotide consists of a phosphate group, a pentose sugar (deoxyribose in DNA or ribose in RNA), and a nitrogenous base.
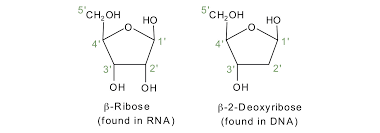
Identify and label the carbons of a pentose sugar
The carbons in a pentose sugar are labeled as 1', 2', 3', 4', and 5', with the 1' carbon attached to the nitrogenous base and the 5' carbon attached to the phosphate group.
Draw the basic structure of a single nucleotide (using circle, pentagon and rectangle)
A nucleotide consists of a circle (phosphate group), a pentagon (pentose sugar), and a rectangle (nitrogenous base), connected in that order.
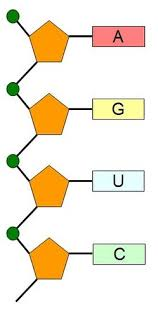
Define “backbone” as related to nucleic acid structure
The "backbone" of a nucleic acid refers to the alternating sugar and phosphate groups that form the structural framework of DNA and RNA.
Explain how nucleotides connect to form a nucleic acid polymer
Nucleotides connect through covalent bonds between the 5' phosphate group of one nucleotide and the 3' hydroxyl group of another, forming a sugar
State the names of the nitrogenous bases found in DNA and RNA
DNA contains adenine (A), thymine (T), cytosine (C), and guanine (G), while RNA contains adenine (A), uracil (U), cytosine (C), and guanine (G).
State a similarity and a difference between the nitrogenous bases
All the four nitrogenous bases are produced from the same molecule inosine. Inosine contains carbon, nitrogen and oxygen atoms which are common to all bases. Differences: Adenine and guanine are purine bases (2 rings) while cytosine, uracil and thymine are pyrimidines bases (1 ring).
Outline how the sequence of bases in a nucleic acid serves as a ‘code.’
The sequence of nitrogenous bases in a nucleic acid determines the order of amino acids in a protein, acting as a code for genetic information.
Define gene
A gene is a sequence of nucleotides in DNA that codes for the synthesis of a specific protein or functional RNA.
Describe the condensation reaction that forms a polymer of RNA from RNA nucleotides
In a condensation reaction, the hydroxyl group (-OH) of the 3' carbon of one RNA nucleotide reacts with the phosphate group (-PO₄) of the 5' carbon of another RNA nucleotide, releasing a water molecule and forming a phosphodiester bond, which links the nucleotides into a polymer.
Identify the monomer and polymer of an RNA molecule
The monomer of an RNA molecule is a nucleotide, while the polymer is an RNA strand or RNA molecule.
Draw a short section of an RNA polymer (using circle, pentagon and rectangle)
Describe the structure of a DNA double helix
A DNA double helix consists of two long strands of nucleotides running in opposite directions, coiled around each other. The strands are connected by hydrogen bonds between complementary nitrogenous bases, forming a helical shape.
Outline the complementary base pairing rule, including the type and number of bonds between bases
In DNA, adenine (A) pairs with thymine (T) through two hydrogen bonds, and cytosine (C) pairs with guanine (G) through three hydrogen bonds.
Define antiparallel in relation to DNA structure
Antiparallel refers to the opposite orientation of the two strands of DNA; one strand runs 5' to 3' while the other runs 3' to 5'.
Compare and contrast the structures of DNA and RNA
both made of nucleotides, polymers, both C pairs with G
DNA double stranded, RNA single stranded
DNA has Thymine, RNA has uracil
DNA has reoxyribose as pentose sugar, RNA has ribose
Compare and contrast the functions of DNA and RNA
DNA stores genetic information and provides instructions for protein synthesis. RNA acts as a messenger (mRNA), transfer (tRNA), and ribosomal component (rRNA) in protein synthesis.
Compare and contrast the location of DNA and RNA in prokaryotic and eukaryotic cells
In prokaryotic cells, DNA is located in the nucleoid region, while RNA is found in the cytoplasm. In eukaryotic cells, DNA is located in the nucleus, while RNA is found in both the nucleus (mRNA, rRNA, tRNA) and the cytoplasm (where protein synthesis occurs).
State the three parts of the cell theory
All living organisms are composed of one or more cells. 2) Cells basic unit of life 3) All cells arise from pre existing cells
Compare the use of the word theory in daily language and scientific language
In daily language, "theory" often means a guess or speculation. In scientific language, "theory" refers to a well proven explanation of a scientific idea which are tested and accepted by the wider scientific community.
Distinguish inductive from deductive reasoning
Inductive reasoning involves drawing general conclusions from specific observations or data. (hypothesis, experimentation, theory) Deductive reasoning involves applying general principles or theories to specific cases to predict outcomes or make conclusions (theory, experimentation, specific hypothesis)

Outline the process of inductive reasoning that led to the development of the cell theory
Inductive reasoning involved observing and documenting various living organisms, noting that they were all made of cells. Repeated observations across different organisms led to the generalization that all living things are composed of cells, forming the basis of the cell theory.
Outline how deductive reasoning can be used to predict characteristics of a newly discovered organism
Deductive reasoning involves applying established principles or theories, such as the cell theory, to make predictions about the characteristics of a new organism. For example, if the cell theory is applied, one could predict that the newly discovered organism will be composed of cells and that these cells will have structures and functions consistent with known cellular principles.
How do we calculate the total microscope magnification given the magnification of the ocular and objective lenses
Total microscope magnification is calculated by multiplying the magnification of the ocular lens by the magnification of the objective lens (e.g., 10x ocular lens and 40x objective lens = 400x total magnification).
Demonstrate how to focus the microscope on a sample
Start with the lowest magnification objective lens and use the coarse focus knob to bring the sample into rough focus, use fine focus to make as clear as possible. Switch to higher magnifications and use the fine focus knob to achieve a clear image.
Demonstrate how to make a temporary wet mount and stain a microscopic sample
Place a drop of water on a slide, add the sample, and cover with a coverslip. Stain the sample with a dye or stain and observe under the microscope.
Demonstrate how to draw cell structures seen with a microscope using sharp, carefully joined lines and straight edge lines for labels
Use a pencil to sketch the observed cell structures with clear, sharp lines. Use a ruler to draw straight, labeled lines for accurate annotations.
Measure the field of view diameter of a microscope under low power
Place a scale or micrometer on the stage, focus on it using low power, and measure the diameter of the field of view.
Calculate the field of view diameter of a microscope under medium or high power
Use the formula: (Field of view diameter at low power) × (Magnification of low power objective) / (Magnification of medium or high power objective).
State the equation for magnification
Magnification of a micrograph or drawing = Size of the image on the micrograph / Actual size of the specimen.
Compare quantitative and qualitative observations
Quantitative observations involve measurable data such as numbers and quantities. Qualitative observations involve descriptive data that can be observed but not measured, such as color, texture, or behavior.
Define resolution and magnification
Resolution is the ability of a microscope to distinguish between two points that are close together. Magnification is the increase in apparent size of an object viewed through the microscope.
Compare the functionality of light and electron microscopes
Light microscopes use visible light to observe specimens and can view live specimens in colour although has a lower magnification and resolution.
Electron microscopes use electron beams with a much higher magnification and resolution although cannot view live specimens or in colour.
State a benefit of using fluorescent stains to visualize cell structures
Fluorescent stains allow for the visualization of specific cell structures or molecules by emitting light when excited by a specific wavelength, providing high contrast and detailed images.
Outline the process of visualizing specific proteins in cells using immunofluorescence technology
Apply a primary antibody specific to the protein of interest, followed by a secondary antibody conjugated with a fluorescent dye. The protein can then be visualized under a fluorescence microscope. Immunofluorescence staining uses antibodies that are conjugated to fluorescent probes to specifically target a chosen protein with such antigens.
Outline the process of producing images of cell surfaces using freeze-fracture electron microscopy
rapidly free a sample
use a steel blade to fracture the sample at the weakest point
some ice is removed
platinum vapour is attatched to the surface, creating a replica of the surface
view this replica using an eletron microscope
Outline the process of visualizing specific proteins using cryogenic electron microscopy
Rapidly freeze the specimen to preserve its native state
view under an electron microscope which is connected to a computer
computer looks for patterns through many different images
can see individual atoms and so structure of specific protein can be visualised
Outline the function of structures that are common to all cells
All cells have a plasma membrane that controls the entry and exit of substances, cytoplasm where metabolic reactions occur, ribosomes for protein synthesis, and DNA as the genetic material that directs cell activities.
Outline the functions of the following structures of an example prokaryotic cell: cell wall, plasma membrane, cytoplasm, 70s ribosome, and nucleoid DNA
The cell wall provides structural support and protection. The plasma membrane controls the movement of substances in and out of the cell. The cytoplasm is the site of metabolic processes. The 70s ribosomes synthesize proteins. The nucleoid DNA contains the genetic information and controls cellular activities.
Define the term “naked” in relation to prokaryotic DNA
"Naked" refers to prokaryotic DNA that is not associated with histone proteins, unlike eukaryotic DNA which is packaged with histones.
Compare and contrast prokaryotic and eukaryotic cell structure
Prokaryotic cells lack a nucleus and membrane-bound organelles (not compartmentalised) and are also smaller
both cytoplasm, cell membrane and DNA and ribosomes although prokaryotes have 70s ribosomes and eukaryotes have 80s (larger) ribosomes
Label a diagram of a eukaryotic cell
A typical eukaryotic cell diagram includes labeled structures such as the plasma membrane, nucleus, mitochondria, chloroplasts (in plant cells), endoplasmic reticulum, Golgi apparatus, and ribosomes.
Outline the function of the following structures in the eukaryotic cell: plasma membrane, cytoplasm, 80s ribosomes, nucleus, mitochondria, chloroplast, endoplasmic reticulum, Golgi apparatus, vesicles, vacuoles, lysosomes, cytoskeleton of microtubules and microfilaments
The plasma membrane controls entry and exit of substances.
The cytoplasm is the site of metabolic reactions.
The 80s ribosomes synthesize proteins.
The nucleus contains genetic material and controls cellular activities.
The mitochondria produce ATP through cellular respiration.
The chloroplast (in plant cells) carries out photosynthesis.
The endoplasmic reticulum transports and synthesises materials (smooth ER for lipids, rough ER for proteins and synthsises ).
The Golgi apparatus modifies, sorts, and packages proteins and lipids.
Vesicles transport materials within the cell.
Vacuoles store substances.
Lysosomes contain enzymes for digestion.
The cytoskeleton (microtubules and microfilaments) provides structural support and facilitates movement.
List the common processes carried out by all life
Metabolism, homeostasis, excretion, growth, nutrition, movement, reproduction, and response to stimuli.
Define metabolism, homeostasis, excretion, growth, nutrition, movement, reproduction, and response to stimuli
Metabolism: all chemical reactions within an organism. Homeostasis: maintaining a stable internal environment. Excretion: removal of waste products. Growth: increase in size or number of cells. Nutrition: obtaining and using materials and energy. Movement: change in position or location. Reproduction: production of offspring. Response to stimuli: reaction to environmental changes.
Describe characteristics of Paramecium or Chlamydomonas that enable it to perform the functions of life
Paramecium
metabolism - cytoplasm for metabolic reactions
reproduction - binary fission
homeostatis - contractile vacuoles to remove excess water
growth - increases cell size
response - cilia to move to respond to stimuli
excretion - waste excreted through anal pore
nutrition - feeds on smaller organisms
Chlamydonas
metabolism - cytoplasm for metabolic reactions
reproduction - asexually or sexuallly
homeostatis - contractile vacuole to remove excess water
growth - increases in cell size
response - eyespot to sense light and move towards it using a flagellum
excretion - excetes waste substances
nutrition - photosynthesis for nutrition
Compare and contrast the structures of plant, animal, and fungal cells with reference to cell walls, vacuoles, chloroplasts, centrioles, cilia, and flagella
Plant cells have cell walls made of cellulose, large central vacuoles, and chloroplasts for photosynthesis. They lack centrioles, but some plant cells may have cilia or flagella. Animal cells lack cell walls and chloroplasts, have small vacuoles, and contain centrioles. Some animal cells have cilia and flagella for movement. Fungal cells have cell walls made of chitin, vacuoles, and no chloroplasts. They do not have centrioles, cilia, or flagella in most cases.