sugars 1
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
what is the general formula for a carbohydrate?
(C.H2O)n
name the 4 general features of carbohydrates
at least one (often 2 or more) chiral carbon
can exist in either linear or ring structure
can form polymer via glycosidic bond formation
can hydrogen bond in water (or other molecules)
what is the general formula for the number of stereoisomers a carbohydrate will have?
2n, where n= no. of chiral centres.
eg aldohexoses have 4 chiral centres and 16 stereoisomers
what forms of carbohydrates are most common (stereoisomers and aldose vs ketose)? and what is a fisher projection?
most sugars are D-isomers.
aldoses (C=O at end) are more common than ketoses (C=O in between two R groups)
fisher projections ignore ability to form cyclic structures, show sugars in linear forms
alcohols readily react with aldehydes and ketones. describe the products of these reactions.
hemiacetals and hemiketals. these have their C=O bonds turned into C-OH and the same chiral carbon will gain a -O-R bond from the alcohol
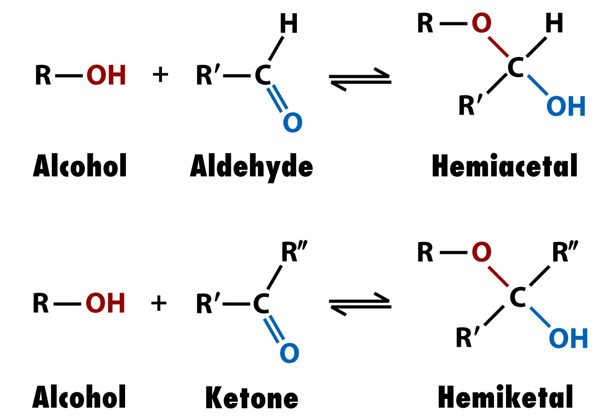
describe a haworth projection, the different types you get, and how to make them from Fisher projections.
haworth projection shows the cyclic structure you get when the OH group of a sugar bonds with the C=O to make hemiacetals or hemiketals
pyranose (hexagonal, 5C ring+ O) and furanose (pentagonal, 4C ring +O)
rotate Fisher projection clockwise 90 degrees, and form a bond by giving H from OH to C=O
iwhen C=O is C1, then if C-OH is C5, you get pyranose, but if its C4 you get furanose
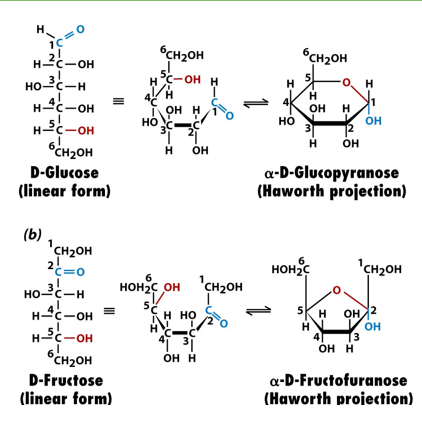
why is it that for both pyranose and furanose, you can get an alpha or beta version?
because there are two sides of the carbonyl plane which the carbon can be attacked from
how many forms of glucose exist in equilibrium in solution? name them. which is most common?
alpha glucopyranose- 2nd most common
beta glucopyranose- most common
alpha glucofuranose
betaglucofuranose
linear D-glucose
different sugars will have different structural preferences to avoid substituents from being too close to each other.
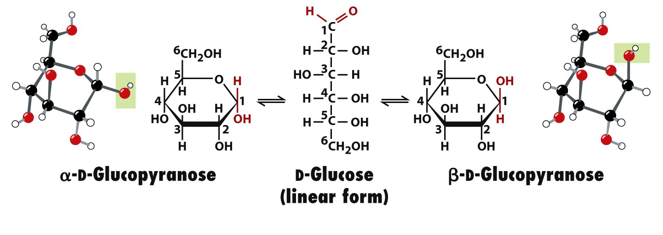
what do equatorial and axial mean? what is the significance of these for sugars? why
equatorial- in plane, axial=perpendicular (less distant from each other)
sugars are more stable if substituents are more in equatorial than axial positions, more distant=less clashes=more stable. chair conformations are slightly more stable than boats for this reason
beta D glucopyranose form is very stable because it allows all bulky groups to be in equatorial positions
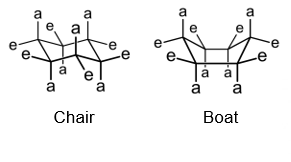
how many possible chair conformations exist for typical pyranoses?
2
what types of sugars are reducing sugars, and what is the product of this reduction? name a solution used to test for reducing sugars and the positive result
aldose sugars are good reducing sugars. any sugar that can reduce an oxidising agent, including ketoses are reducing sugars though
aldonic acids from aldoses (HC=O becomes COOH)
Fehling’s solution (alkaline CuSO4), produces a red precipitate (Cu2O)
why does D-glucopyranose not act as a reducing sugar?
it is rarely ever monomeric, so rarely has a free C=O bond to act as a reducing agent
sometimes sugars can be aminated like alpha-D-glucosamine. what are these important for?
molecular recognition, act as building blocks for oligosaccharides. not important in metabolism like normal glucose
how and why do sugar structures change in solution?
interchange between linear and ring form until polymers form
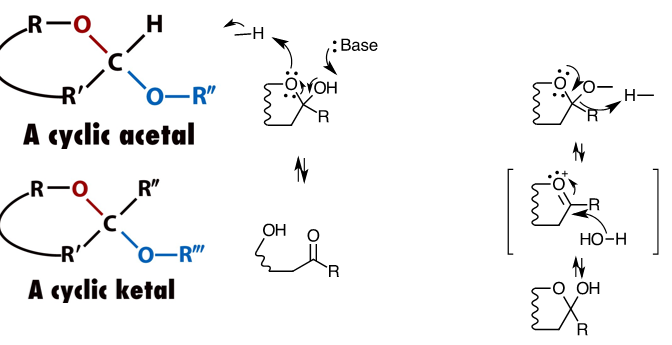
OH groups group in cyclic acetals and ketals have rapid equilibrium with open form
but when OR bond is made instead, this no longer happens since that would require oxocarbenium ion formation (difficult)
thus monomers will be trapped in a given configuration once polymerised
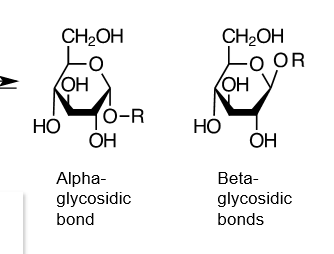
true or false: alpha glycosidic linkages are similar to beta
false. they will make very different oligosaccharides, and are not even interchangeable via enzymatic conversion. you can tell which form is being made depending on whether the glucose on the left is alpha or beta
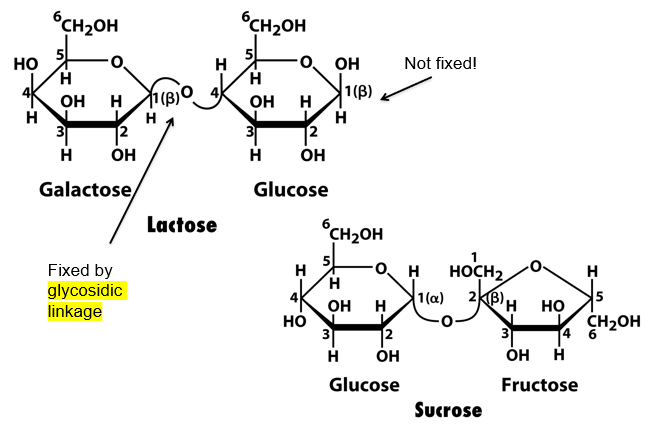
compare the effect of sucrose on blue Fehlings solution to that of sucrose. explain
sucrose will not change the colour, while lactose will make red precipitate
this is because lactose has a beta glycosidic linkage, meaning its monomer has an OH group that can form an aldehyde via ring open chemistry. this aldehyde can reduce Fehling’s
sucrose doesnt have this
the glucose monomer of sucrose cannot ring open since the the oxygen that rings open to make C=O is used up in the glycosidic link. the OH group on the left of the glucose cant make C=O
describe the polymer of beta glucose
cellulose.
all substituents are equatorial, so you can make sheets where all monomers are in the same plane.
sheets can be layered.
cellulose is crystalline and insoluble. equatorial positions mean that hydrogen bonds dont form with water but between monomers
describe polymers of alpha glucose
amylose/ amylopectin
digestible, unlike cellulose
linkage prevents ring opening of glucose and provides insolubility