MICR 270 Mod 5 - Vaccines & Translational Immunology
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
Enzyme-Linked Immunoabsorbent Assay (ELISA) (how does it work?)
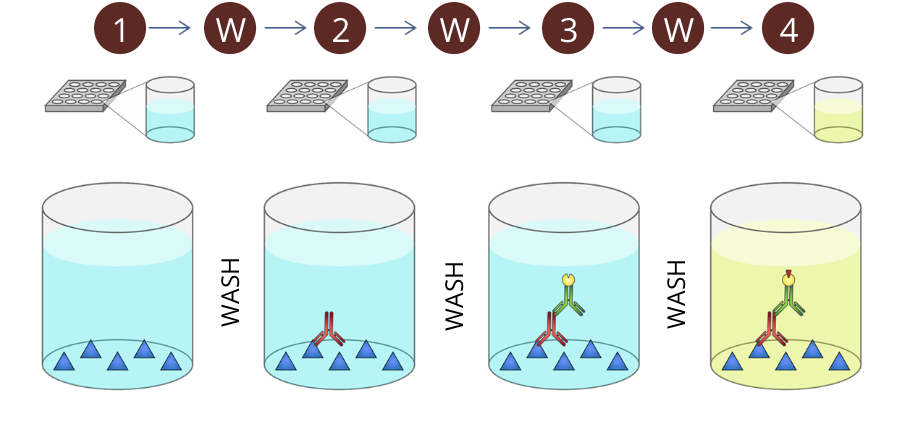
ELISA is a fundamental tool of clinical immunology based on the principle of antigen-antibody interaction
the bottom of the wells are coated with an antigen that is specifically recognized by the antibody you wish to measure (primary antibody)
W - the wells are washed to remove any excess antigen not attached to the bottom of the well
the sample containing the antibody to be measured (ie. a patient’s serum) is added to the well. The primary antibodies, if present, will bind to the antigens attached to the bottom of the well
W - the wells are washed again to remove excess primary antibody not attached to the bound antigen as well as any other sample components that might interfere with subsequent steps
an enzyme-conjugated secondary antibody (a secondary antibody that specifically binds to the primary antibody. In this example, the secondary antibody used has an enzyme attached to it) is added to the well. This secondary antibody will bind to the Fc portion of the primary antibodies already present in the well. The secondary antibody used specifically recognizes antibodies from a specific animal (ie. anti-human, anti-rat, anti-rabit, etc)
W - the wells are washed to remove any excess secondary antibody no attached to the primary antibody
the substrate of the enzyme attached to the secondary antibody is added to the well. the reaction of the substrate (a chromogen, can be readily converted into dye) and the enzyme produces a coloured product that can be measured by absorbance
What does ELISA measure?
an ELISA will measure a coloured reaction product by absorbance with the help of a machine called a spectrophotometric plate reader
the data measured correlates with the presence of an antibody or an antigen
this information can be used, for example, to detect the presence of a viral disease
Indirect ELSA
detects or quantifies antibodies
ie. to determine the presence of serum antibodies against HIV
How does flow cytometry work?
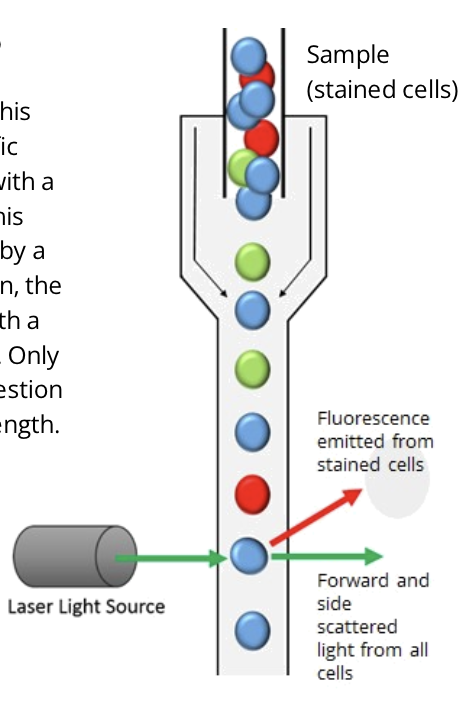
flow cytometry is a method of detecting and quantifying different cell types in a mixed cell suspension
a narrow stream of cells in single file is passed through a laser light source
the way laser light is scattered is unique to each cell type; this can be detected and analyzed'
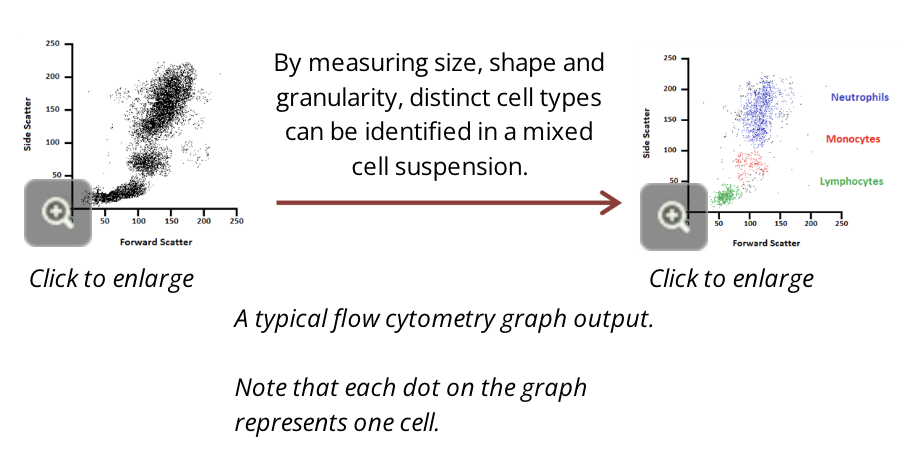
measuring FSC allows for the discrimination of cells by size
FSC intensity is proportional to the diameter of the cell
SSC provides information about the internal complexity (ie. granularity) of a cell
for example, granules and the nucleus increase the side scatter of the laser in a flow cytometer
when measured in conjunction, FSC and SSC allow for the detection of specific cells and cellular components within a heterogeneous population
flow cytometry can also be used to determine the proportion of cells expressing a particular antigen
in this case, cells are labelled with a specific antibody
the antibody is coupled with a fluorescent marker. this marker can be excited by a light of a specific wavelength
in turn, the fluorescent marker emits a light with a characteristic different wavelength
only cells expressing this antibody in question will emit light of this specific wavelength
what does flow cytometry measure?
flow cytometry is used to measure physical properties of a cell
it can also be used to detect specific antigens on or inside a cell
as each cell in a mixed suspension is assessed as an individual detection event, the total number of cells in the suspension, the number of cells of a particular type in the suspension, and the overall composition of the suspension can be readily determined
flow cytometers are used to determine complete blood counts
clinical application of flow cytometry
flow cytometry can be used to diagnose cancer in a variety of ways
the main diagnostic tests focus on detecting DNA aneuploidy (abnormal number of chromosomes), analyzing cell cycles, and the immunophenotypical characterization
how do monoclonal antibodies work?
monoclonal antibodies are antibodies that are produced by a single clone of a B cell that are specific for a single epitope (the portion of the antigen that is recognized and bound by an antibody)
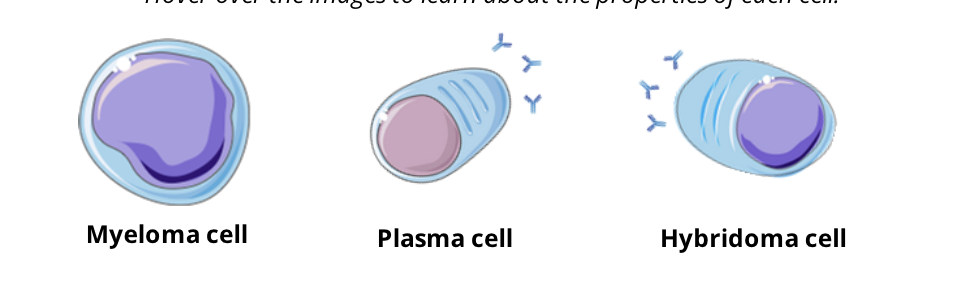
monoclonal antibodies are produced in the lab by hybridomas, immortal cells that produce unlimited quantities of one identical antibody
hybridomas are the result of fusion between a plasma cell and a cancerous (or myeloma) cell
hybridomas share properties of both plasma cells and myeloma cells
Myeloma cell: immortal growth, divides indefinitely
Plasma Cell: produces specific antibodies against one antigen
Hybridoma cell: a perpetual source of antibodies against one antigen
clinical applications of monoclonal antibodies
Immunotoxins
immunotoxins consist of a tumour-specific monoclonal antibody attached to a deadly toxin
this technique is still under investigation, but a long-term objective is to use immunotoxins to target and eliminate tumour cells and treat cancer
Radiolabelled Antibodies
monoclonal antibodies tagged with a radioactive isotope can be used to diagnose tumours earlier that other methods
radiolabelled antibodies can bind to antigens on a tumour thereby allowing the precise location of the tumour within the body to be visualized
Live-Attenuated Vaccine
Characteristics
contains a modified strain of the disease causing agent which has lost its pathogenic ability, but retains its capacity to replicate within the host
Advantages
provides a prolonged exposure to the disease causing agent, and its suitable to generate cell-mediated immunity
Disadvantages
potential to revert to a virulent form
requires specific storage and transport conditions (ie. refrigeration)
Examples
smallpox vaccine
oral poliovirus vaccine
measles vaccine
Killed-Inactivated Vaccine
Characteristics
contains a strain of the disease causing agent that has been inactivated by heat, chemicals, or radiation
has the ability to generate an immune response, but is unable to replicate
Advantages
safer option as it cannot mutate back into virulent form
easy to store and transport
Disadvantages
generally requires multiple booster doses to maintain immunity
generally must be administered by injection
Examples
rabies vaccine
influenza vaccine
Toxoid Vaccine
characteristics
contains an inactivated toxin which is a product from the pathogen that is causing the disease
Advantages
safe as it is not a living organism that can divide, spread, and/or revert
stable as they are less susceptible to changes in temperature, humidity, and light
Disadvantages
may require several doses and usually need an adjuvant (a substance that enhances the body’s immune response to an antigen)
Examples
tetanus vaccine
diphtheria vaccine
Subunit Vaccine
Characteristics
contains only a small part or fragment of the disease causing agent
Advantages
the safest type of vaccine - can be used on everyone including immunocompromised, pregnant, or elderly populations
Disadvantages
rarely successful at inducing long lasting immunity, which means it will require multiple booster doses to maintain immunity and might even need to be conjugated to a carrier
a carrier is a stronger antigen that the desired target antigen. By covalently attaching a strong antigen to a poor antigen, the overall immunological response is strengthened, and hopefully the immunological response to the poor antigen is also improved
Examples
Hepatitis B vaccine
mRNA Vaccines
mRNA vaccines are the most recent vaccine type that have changed the field of vaccinology
they are known for their use against SARS-CoV-2, the COVID virus, and are used in several formulations to fight the virus (ie. bivalent vaccine, boosters)
formulations of mRNA vaccines are also being investigated for other infectious diseases (ie. HIV, influenza), and non infectious clinical conditions (ie. pancreatic cancer, heart tissue regeneration)
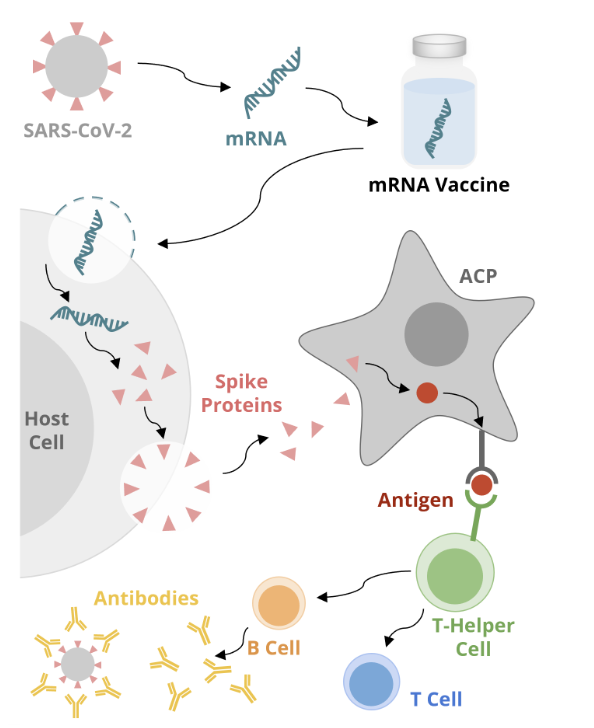
the principle of the assay relies on the use of mRNA to produce viral proteins and recruit immune cells to respond to the antigenic target
the proteins are then displayed on the surface of an antigen presenting cell to induce B cells and T cell immunity
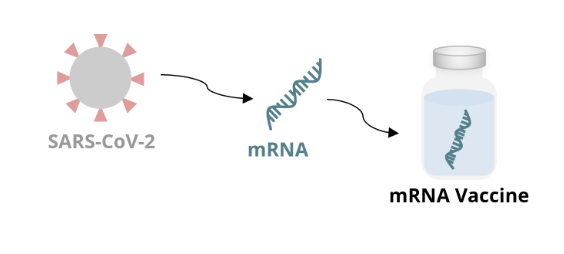
mRNA vaccine mechanism: vaccine production
mRNA is made in the lab from a DNA template of the virus
the mRNA encodes an antigen of the virus
for the COVID-19 vaccine, the mRNA encodes the spike protein on the surface of the virus
the mRNA is incorporated into a formulation that can be administered as a vaccine
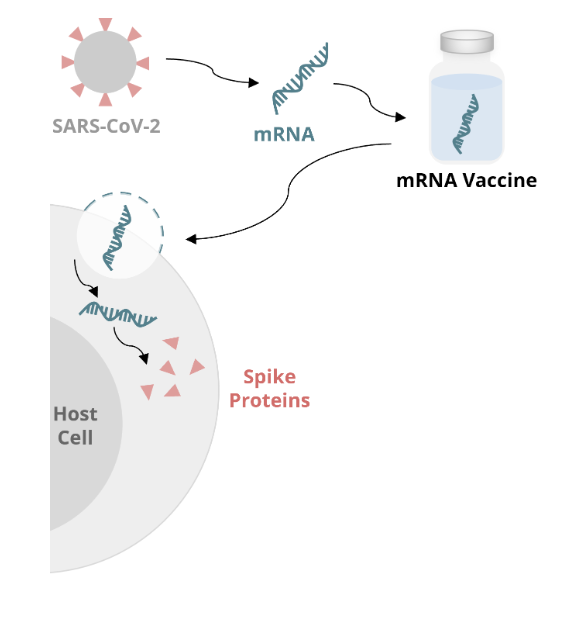
mRNA vaccine mechanism: host cell
once inside the body, the mRNA enters the host cell and uses host cell machinery to produce the spike protein
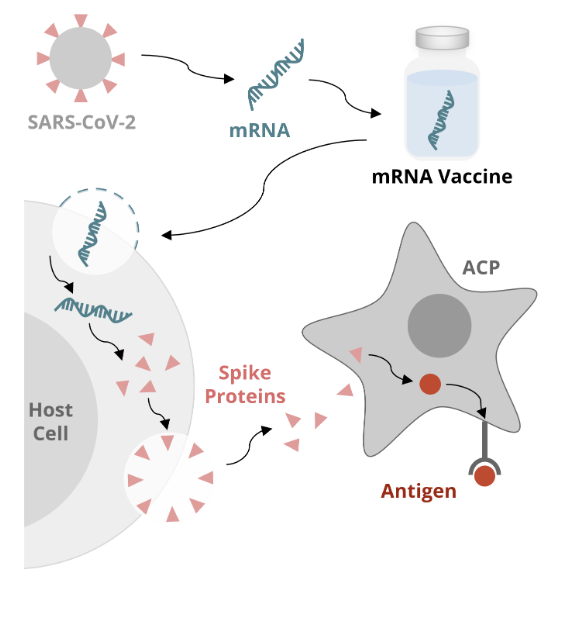
mRNA vaccine mechanism: APC
the newly formed spike protein exits the cell and is recognized by an APC
the APC internalizes the spike protein and processes it into a peptide (or antigen)
the APC then displays the antigen on the surface of the cell via the major histocompatibility complex
mRNA vaccine mechanism: immune response
the antigen is recognized by a helper T cell, which initiates an immune response
B cells produce antibodies that stop the virus from infecting cells
T cells destroy cells infected with the virus
antiviral medications against COVID-19
Polymerase Inhibitor
polymerase is an enzyme that plays a central role in viral replication and transcription
Molnupiravir is a polymerase inhibitor used to treat COVID 19
it increases the frequency of viral RNA mutations and impairs replication of the virus
Protease Inhibitor
proteases cut proteins into smaller, more workable pieces
protease inhibitors are often administered in combination
for example, nirmatreIvir stops protease from cutting viral proteins into functional pieces
Ritonavir protects nirmatreIvir from destruction by the body and allows it to keep working
since these drugs disrupt the assembly of the virus, they can’t replicate and infect other cells
HPV VLP Vaccines
VLPs (virus like particles) are composed of the structural proteins of HPV, which can self assemble into particles that resemble the natural virus both structurally and immunologically
because they do not contain viral DNA, VLPs are not infectious
Ebola Vaccines
Ebola virus (EBOV) and Marburg virus (MARV) produce transmembrane glycoproteins thought to play a role in the virulence of these viruses
researchers have developed a potentially viable vaccine using glycoproteins from EBOV and putting them into a live attenuated recombinant vesicular stomatitis virus (VSV), that expresses the transmembrane glycoproteins of EBOV and MARV
Genital Herpes Vaccine
a subunit vaccine was developed which was composed of a prominent structural protein of HSV-2
this vaccine showed promise in early clinical trials, with reported efficacies of >70% in women who were seronegative for both HSV-1 and HSV-2 at the beginning of the trial
a subsequent larger clinical trial however showed much lower efficacy (20%) and no protection from infection by HSV-2

phases of vaccine development: lab studies
the first step in vaccine development is to identify the infectious agent causing the disease and select a strain (or subtype) which is relevant to the target population and will be used to produce the vaccine
this stage is largely dependent on research carried out in the lab using assays, which may involve exhaustive screening to identify a suitable antigen and creation of a vaccine concept
this stage also involves developing and testing the manufacturing process of the vaccine according to good manufacturing process standards
phases of vaccine development: preclinical studies
preclinical studies involve research carried out in animal models to evaluate the pharmacological aspects of the product
this is a stage of research that occurs before the clinical trials can begin
at this point, the researchers will carry out challenge studies to demonstrate the immunogenicity of the vaccine in animal models
immunogenicity means that the vaccine has the ability to induce an immune response which will prevent the development of the disease in case of subsequent infection with the pathogen
another part of this stage is to carry out safety studies to evaluate the possible toxicity of the vaccine which would prevent its use in humans
phases of vaccine development: clinical phase I
due to rigorous regulatory requirements, only a very small percentage of vaccines progress to licensing, making the costs of vaccine research and development extremely high
Phase I clinical trials for vaccines involve small scale trials in humans (10-<100 people) to assess vaccine safety by evaluating local and systemic reactions after administration
it also provides preliminary data on the immunogenicity of the vaccine and the immune response it evokes
phases of vaccine development: clinical phase II
phase II trials occur at a bigger scale (50-500 people) to collect data on safety, side effects, and the efficacy of the vaccine
this stage also evaluates the dosage requirements of the vaccine
clinical trial evaluators monitor the effects of increasing vaccine dosage and conduct challenge tests to define the optimal dose and vaccination schedule (ie. need for boosters) in target populations
phases of vaccine development: clinical phase III
phase III trials involve multiple geographic sites with many hundreds of subjects (300-30,000 people) to evaluate efficacy under natural disease conditions
in this step, researchers are required to demonstrate the efficacy in target populations and complete a safety assessment for the vaccine
if the vaccine is successful in retaining safety and efficacy over a defined period of time, the manufacturer will then apply to the regulatory authorities for a license to market the product for human use
phases of vaccine development: health canada approval
health canada is the regulatory authority in canada responsible for ensuring the quality, safety, and efficacy of all biological drugs, including vaccines for human use
vaccine regulation is necessary as they are usually given to very large numbers of healthy individuals
vaccines candidates must be submitted to health canada to be considered for approval with sufficient scientific and clinical evidence to show that it is safe, efficacious, and of suitable quality
scientific evidence includes results form human clinical trials
vaccines cannot be used clinically without approval from health canada
challenges with vaccine development
high cost of the development of vaccines sometimes leads to premature abandonment of clinical research
live attenuated vaccines, the most effective at producing long term immunological memory, need to be stored and transported in specific conditions
the efficacy of a vaccine is directly related to specific antigens against which the immune response has developed
if the disease causing agents evolve by changing or losing their major antigenic determinants, the vaccine could lose its efficacy
even if a vaccine has been approved, that doesn’t mean the work is done.
new strategies are developed to create better vaccines, for example, by using recombinant vector or improving vaccine adjuvant
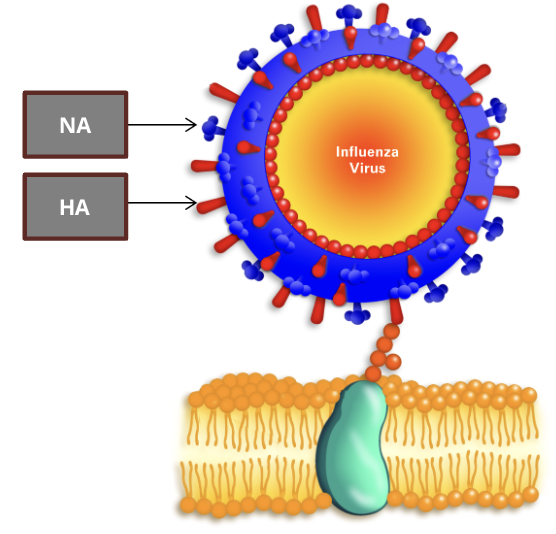
vaccine development challenges: influenza virus
Neuroaminidase Antigen (NA)
a surface protein that removes sialic acid from cell surfaces and enables new viral copies to infect and spread to other cells
researchers have identified 11 NA subtypes, each with sequence variabilities in their receptor binding sites
Haemagglutinin Antigen (HA)
a surface protein that recognizes and binds to sialic acid on cell surface glycoproteins
these HA cell surface interactions lead to endocytosis of the virus, and the HAs are activated to fuse the endosome and viral membrane
researchers have identified 18 HA subtypes, each with sequence variabilities in their receptor binding sites
herd immunity
herd immunity can be measured by calculating what is called the basic reproduction number, R0
R0 is defined as the average number of secondary cases of an infectious disease arising from a typical case in a totally susceptible population
R0 determines the herd immunity threshold and therefore the immunization coverage required to achieve elimination of an infectious disease
as R0 increases, higher immunization coverage is required to achieve herd immunity
introduction to cancer
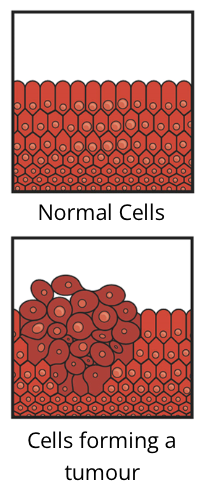
cancer cells vs normal cells
cancer cells behave differently than normal cells of the body
they do not need specific growth factors to divide, which normal cells do
they also don’t respond to the signals that cause normal cells to stop dividing
in the field of immunology, cancer cells are viewed as self cells that have been altered to escape the normal growth-regulating mechanisms
these changes are the results of alterations in DNA which induce cell transformation to malignant cells
Tumour
cancer cells continue to divide and grow, ultimately forming a tumour
a tumour is an abnormal mass in tissue
Cancer Immunotherapy
cancer treatment approaches that are either based on secreted or cellular components of the immune system are broadly defined as immunotherapies
these therapies are primarily aimed at enhancing host anti tumour immune responses and have recently joined the pillars of cancer management
tumours
benign tumour
not cancerous
unable to grow indefinitely or invade surrounding tissues
malignant tumour
cancerous
ability to metastasize
continuous growth
the resilience of cancer cells
cancer cells show resilience in environments that a normal cell might not survive
HeLa Cells
HeLa cells are a line of cancerous cells which were cultured from Henrietta Lacks in 1951
these cells were the first line of human cell culture to survive outside the human body
the resilience of HeLa cells allowed for their immortality, thus the cell cultures are an important tool for scientific discovery to this day
HeLa Cells: research breakthroughs
Recombinant Protein Production
although bacterial cells can be utilized for protein production, they lack the required mechanics to produce more complex proteins. The HeLa cell line allowed researchers to overcome this challenge
HPV Vaccine
researchers found that HeLa cells were infected with HPV-18 virus, and decades of characterizing HPV-18 led to the development of HPV vaccines
Understanding Virology
experimental viral infection on HeLa cells allowed researchers to characterize how specific viruses can evade the immune system, ie. CD4 T cell receptor utilization by HIV
Toxicity Testing
originally, hepatocytes were used to test toxicity, but proved too unstable for sustained use. the resilient HeLa cells are now used to test cytotoxicity of drugs, an important practice in drug development and discovery
Monoclonal Antibody (mAB) production
mABs, which can be produced using hybridoma crosses of HeLa and other animal cells, have many applications such as medical diagnoses and cancer therapy
Polio Vaccine
this vaccine was developed in the 1950s, but HeLa cells were the only human cells that could be used to test the vaccine
Genome Sequencing
HeLa cells fused with mouse cells became the first hybrid cells, the fusion of two cell types, in research. This breakthrough aided in the emergence of the human genome project
Telomerase Activity
in 1996, scientist Gregg Morin isolated telomerase from HeLa cells which were previously only found in animal embryos. This supported his hypothesis that both embryos and cancer cells utilize telomerase to rapidly divide, giving researchers insight on the importance of telomerase in human embryology
the cancer-immunity cycle stage 1: release of cancer cell antigens
cancer cell death
antigens are released by mutated cancer cells, indicating that they are not healthy cells
the immune system is able to recognize these antigens
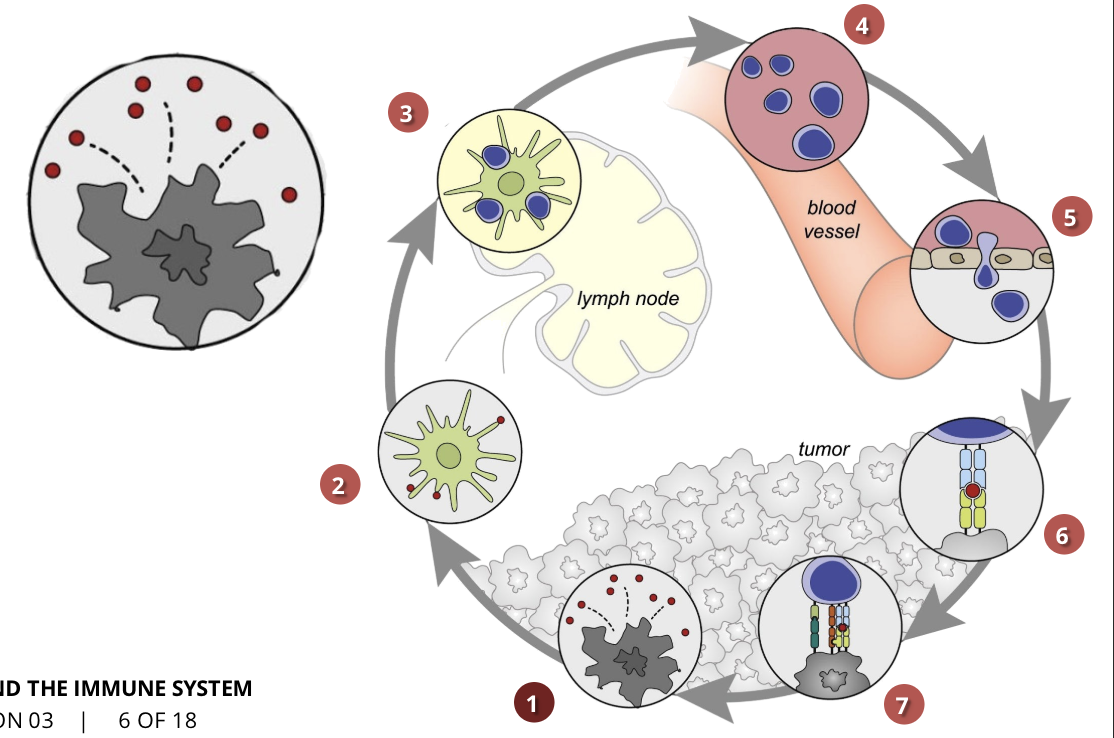
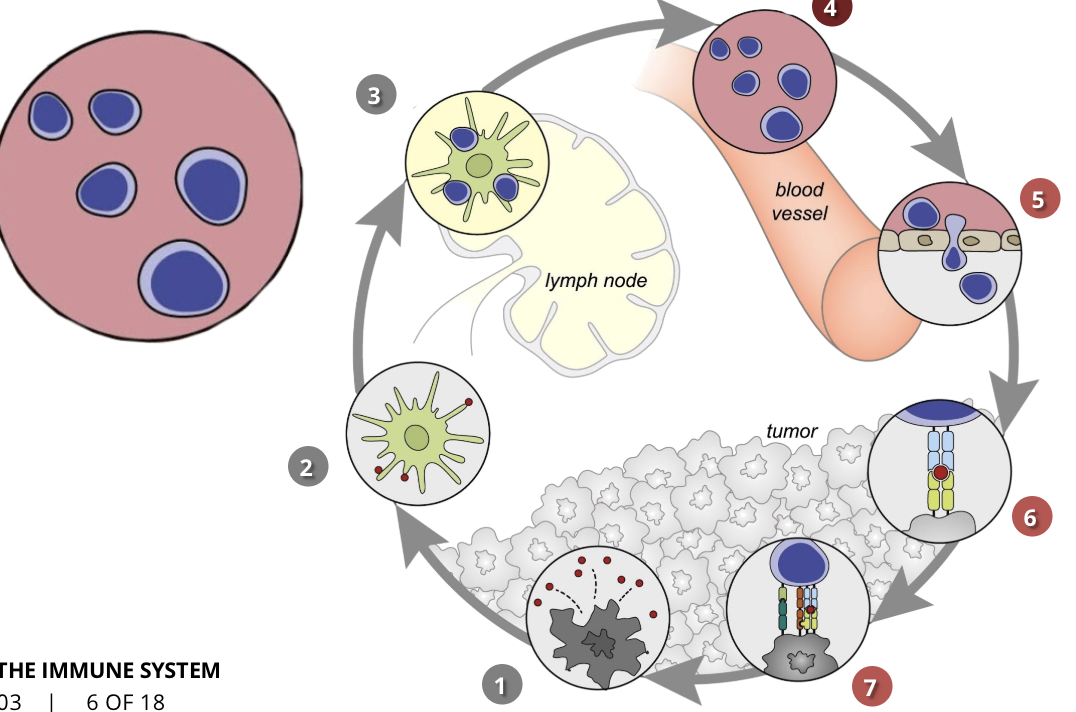
the cancer-immunity cycle stage 2: cancer antigen presentation
dendritic cells/APCs
the cells of the immune system capture the released antigens and travel to the lymph nodes where they find T cells
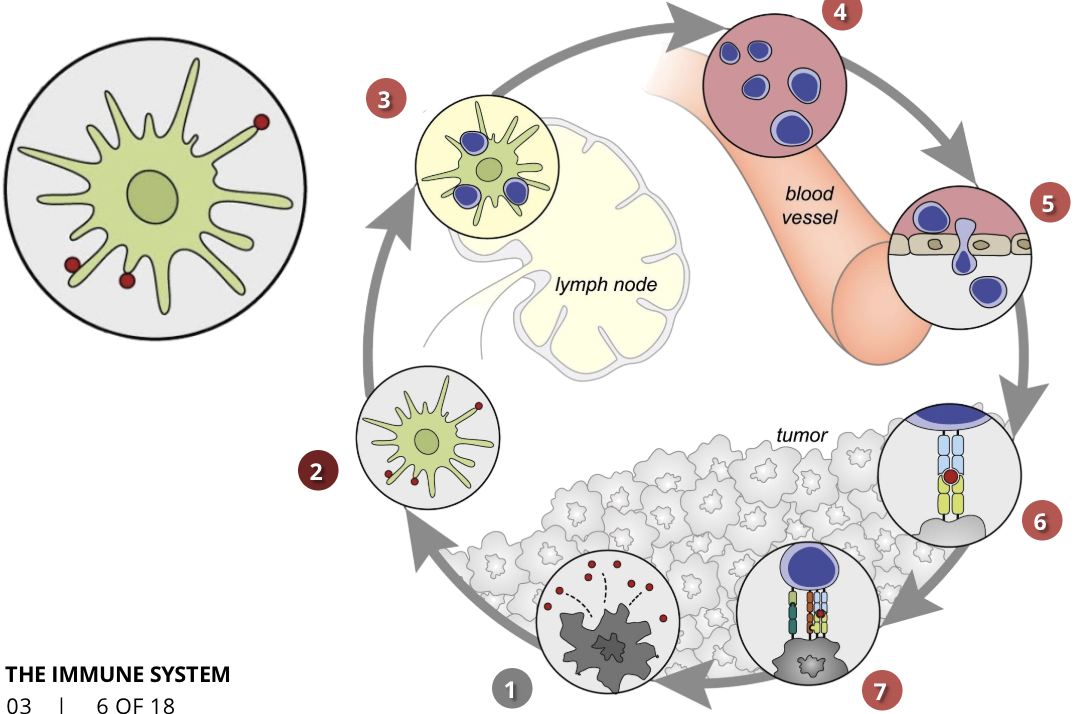
the cancer-immunity cycle stage 3: priming and activation
APCs & T cells
T cells are activated by the antigens and the immune response against the cancer is initiated
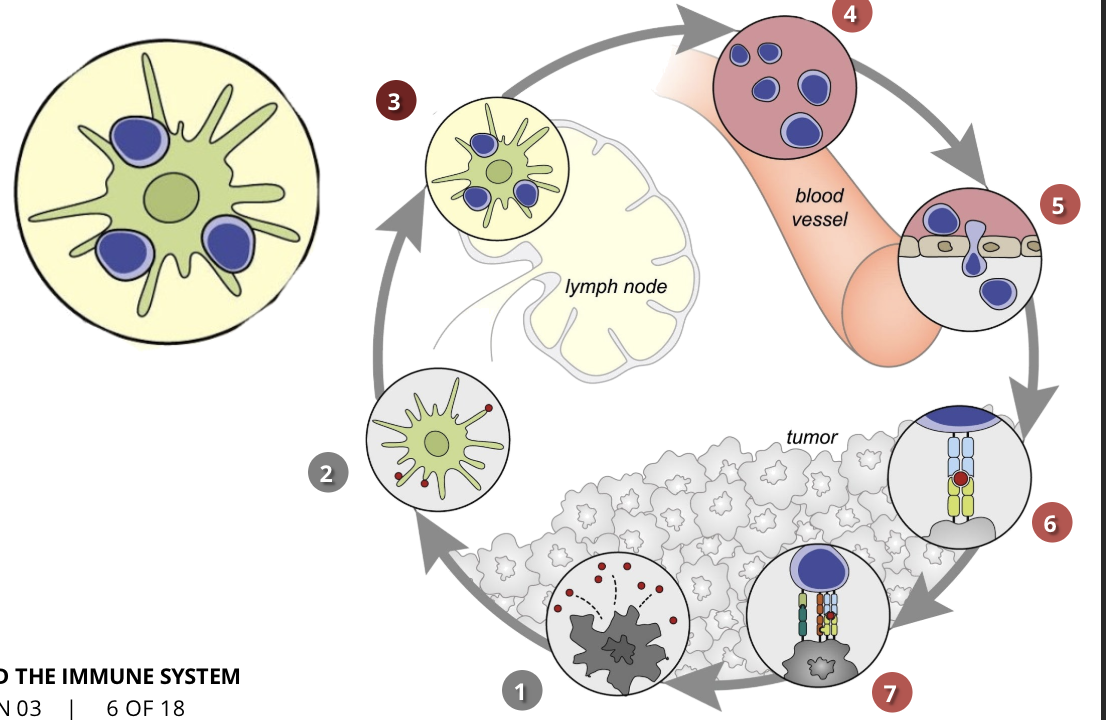
the cancer-immunity cycle stage 4: trafficking of T cells to tumours (CTLs)
the activated T cells move through the blood vessels to the site of the tumour
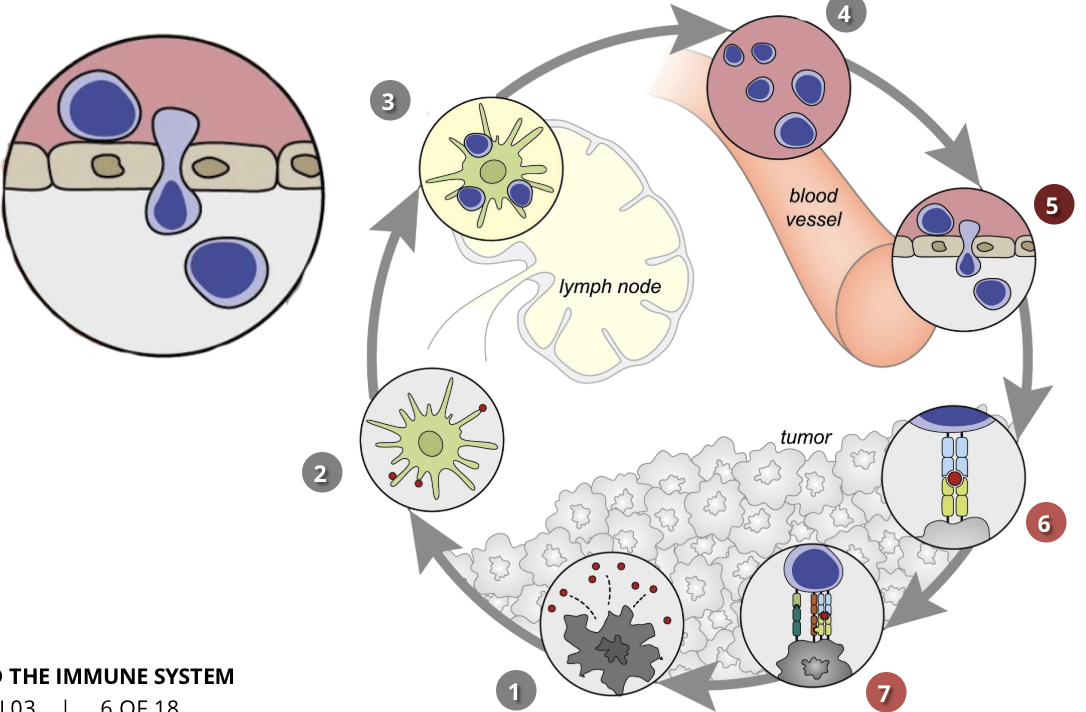
the cancer-immunity cycle stage 5: infiltration of T cells into tumours
CTLs, endothelial cells
once the T cells reach the cancerous cells, they invade the tumour and attack it
the cancer-immunity cycle stage 6: recognition of cancer cells by T cells
CTLs, cancer cells
T cells recognize cancer cells because of the antigens they had previously released
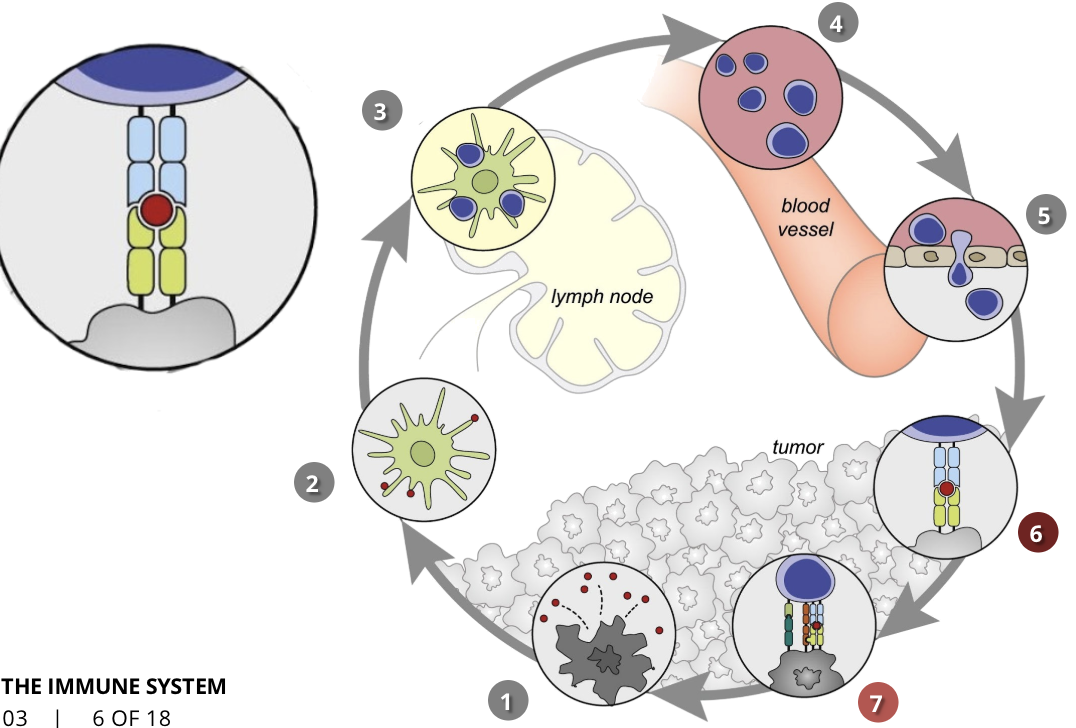
the cancer-immunity cycle stage 7: killing of cancer cells
immune & cancer cells
T cells initiate a pathway that results in cancer cell death
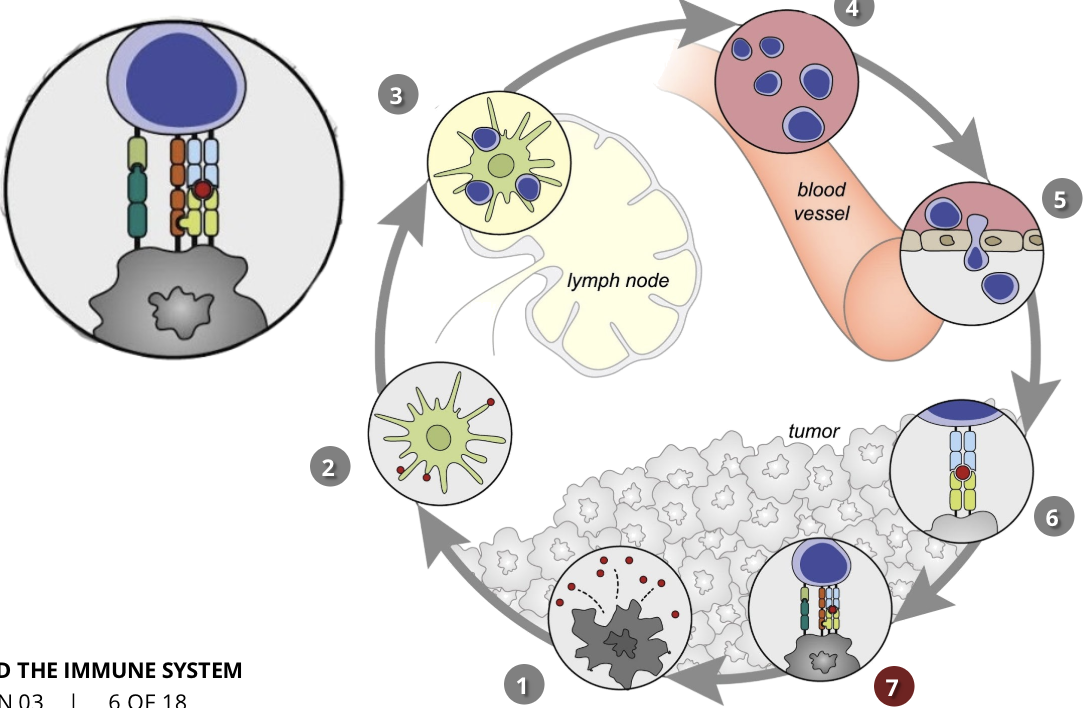
tumour immunosurveillance vs immunoediting
Immunosurveillance
theory of immunosurveillance states that tumour cells are identified and kept under control by the immune systems of healthy individuals
however, cancer cells sometimes evade recognition by the immune system, or the magnitude of the antigen-tumour immune response is not sufficient to kill all of the cancer cells
recent research has even shown that often the immune environment around the cancer cells can promote tumour progression
Immunoediting
immunoediting is. adynamic process which describes the connection between the tumour cells and the immune system in the context of immunosurveillance and tumour progression
during cancer evolution, there is ongoing cross talk between the cancer cells and the immune cells
the immune system destroys the growing cancer cells, but this promotes tumour growth in the cells which have the ability to evade immunosurveillance
cancer immunoediting is constituted by 3 phases:
elimination
equilibrium
escape
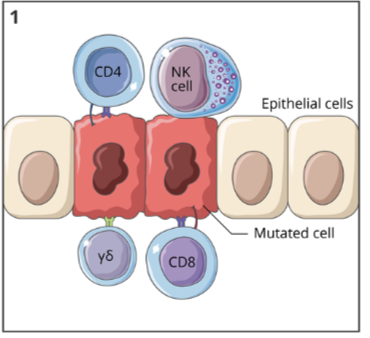
cancer immunoediting: elimination
when a tumour cell arises in a tissue, the immune system can quickly act to remove it
a variety of immune cells, including NK cells, cytotoxic T cells, and helper T cells, can recognize the altered cell and work to eliminate it
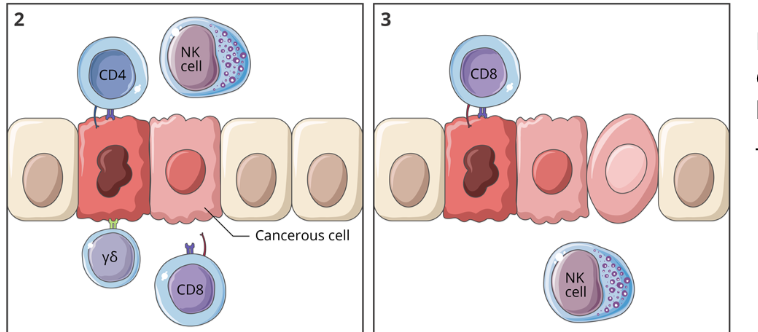
cancer immunoediting: equilibrium
if the tumour cells are not eliminated, they can enter a state of equilibrium where the cell proliferation is matched by cell killing by the immune system
this phase can last for a short time or many years
cancer immunoediting: escape
tumour cells are no longer recognized by the immune system, so avoid elimination
these cells are able to grown uncontrolled and eventually proliferate to form a tumour
cancer evasion of the immune response
reduced MHC expression
tumour cells display low levels of MHC class I molecules on their cell surface
as cytotoxic T lymphocytes (CTLs) recognize antigens in the context of MHC class I molecules, an absence of these molecules will inhibit recognition of tumour cells
Poor Costimulatory Molecules
T cells require both expression of MHC and costimulatory molecules to become activated
tumours lack these costimulatory molecules, which contribute to their poor immunogenicity
T cells will only be partially activated
cancer immunotherapy
immunotherapy is able to attack cancerous cells throughout all organs in the body
immunotherapy allows the immune system to specifically target and eliminate cancer cells without damaging healthy cells, resulting in fewer side effects that traditional cancer treatments
immunotherapy takes advantage of immunological memory, allowing for the possibility of long term protection
immunotherapy can be applied to almost all types of cancer
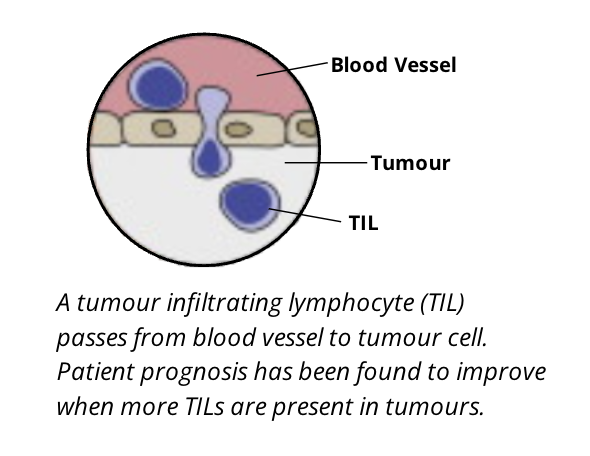
tumour infiltrating lymphocytes (TILs)
although T cells are shown to be the most effective lymphocyte population in killing cancer cells, B cell lymphocytes are also important. these are broadly termed tumour infiltrating lymphocytes
the type, density, and location of TILs has been suggested as. a prognostic biomarker in some cancers
TILs leave the bloodstream and migrate to infiltrate the tumour under influence of various chemotactic gradients of specific types of chemokines
TILs can be a mix of T and B cells. NK cells, dendritic cells, and macrophages also can be of prognostic relevance
it is important to note that the number of TILs is not always reflective of their activity and prognostic significance
some of the dynamic phenotypic markers expressed on these cells reflect their activation status and therefore must accompany any interpretation on their roles as biomarkers for diagnosis, such as in the case of breast cancer
Prognostic biomarker: biological characteristics that are objectively measured and evaluated to predict the course of a disease or response to a therapeutic intervention among patients with the same characteristic
Biomarkers: a measurable substance in an organism, the presence of which is indicative of some phenomenon such as disease, infection, or environmental exposure
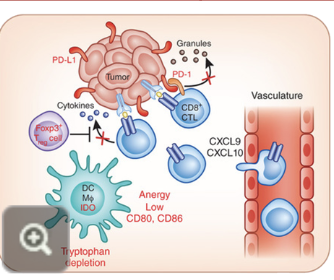
immunological classification of tumours: T cell inflamed “hot” tumours
hot tumours show compartively higher immune activity compared to cold tumours
characteristics:
high numbers of CD8+ TILs
high levels of interferon IFN genes
usually respond well to treatment (chemo or immunotherapy)
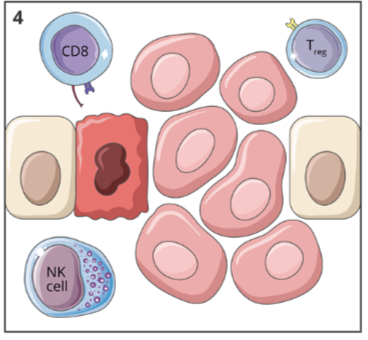
immunological classification of tumours: T cell non inflamed “cold” tumours
cold tumours show lower immune activity compared to hot tumours
characteristics:
low numbers of CD8+ TILs
low levels of interferon (IFN) genes
usually inferior response to treatment (immunotherapy or chemo)
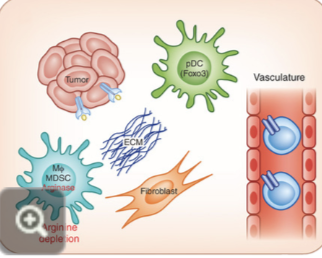
treatment of cold and hot tumours
because of this immunological difference between hot and cold tumours, conceptually, one could convert cold tumours. to hot by stimulating the tumour interferon activity
this knowledge has led to the exploration of anti tumour immunity towards development of newer treatments
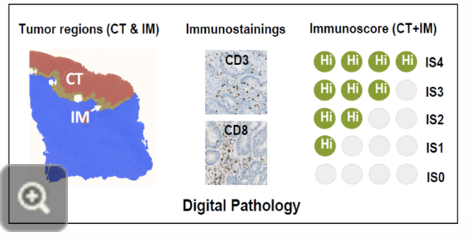
the immunoscore
combining the knowledge of TILs and the immunological classification of hot and cold tumours has led scientists to develop a new and reliable prognostic biomarker for cancer diagnosis, such as in the case of colon cancer
an immunoscore measures the density/numbers of T cells in the centre (CT) and in the periphery (IM) of the tumour by immunochemistry
this can help stratify patients as having high risk or low risk cancers, and aid in developing treatment plans
Workflow for determining an immunoscore:
separating the tumour in the central (CT) and peripheral (IM) regions
staining for T cells and conducting digital pathology
assigning a score to the tumour to relate it with an associated diagnosis or risk attribution
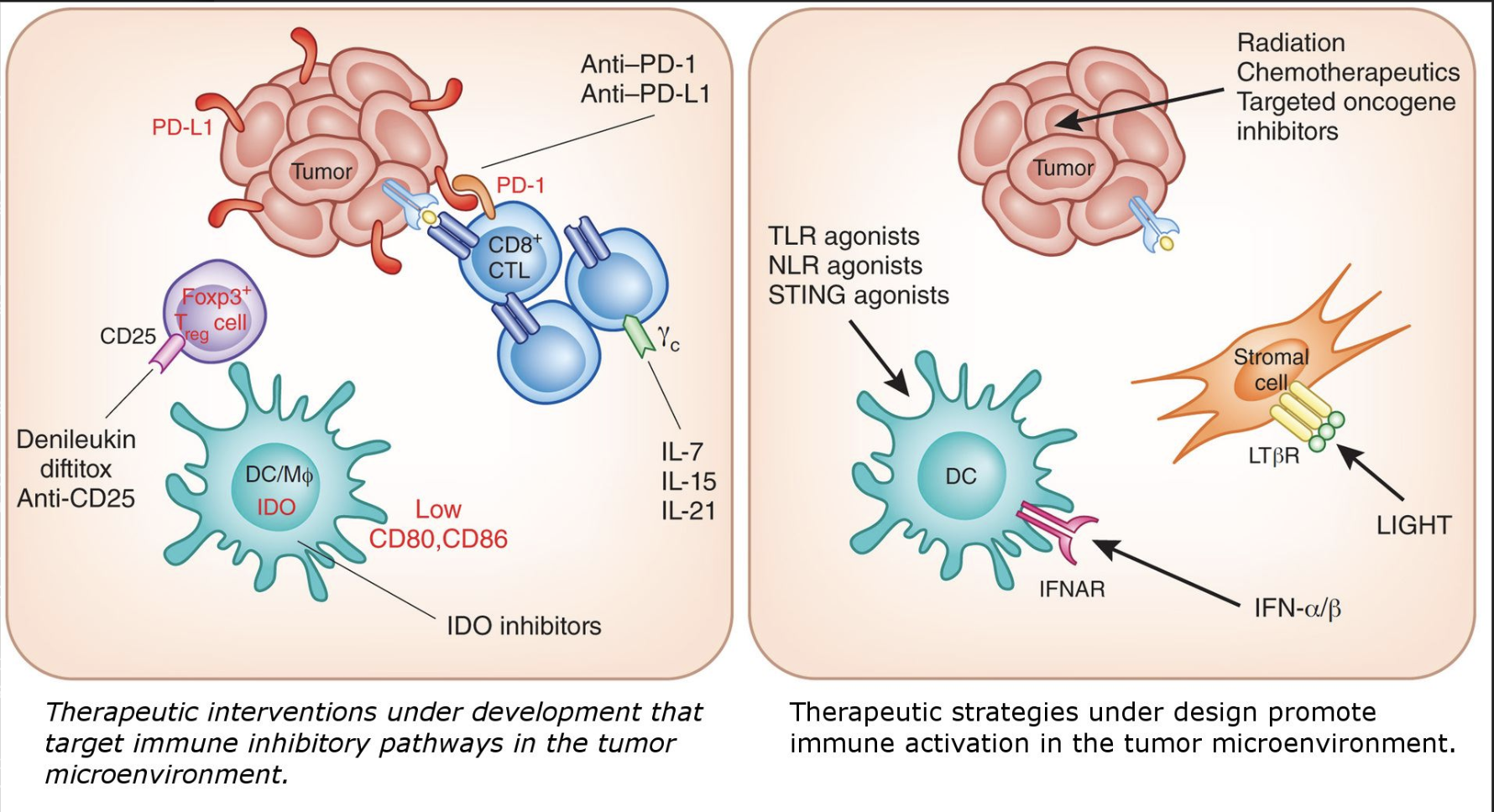
immunotherapy approaches based on tumour immune microenvironment
the immune system can be activated through a variety of ways to combat tumours
there are many other therapies under development that exploit the components of the immune system to fight cancer, and the field is constantly innovating and developing solutions to this complex health issue