BSCI330: Intracellular Transport!
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
44 Terms
What is the cytoplasm?
cytosol (protein synthesis and degradation) + organelles
Where does gated transport happen?
Between cytosol and nucleus!
Bidirectional! Topologically similar compartments.
What is transmembrane transport?
Between cytosol AND nucleus, peroxisomes, plastids, mitochondria, endoplasmic reticulum.
Unidirectional! Topologically different compartments.
What is vesicular transport?
ER to Golgi
Golgi to endosomes, lysomes, secretory vesicles, and cell exterior.
Bidirectional! Topologically similar compartments!
What does topologically similar mean?
Compartments with similar membrane orientations.
Which side of the membrane you are on. Different side of membrane or same side?
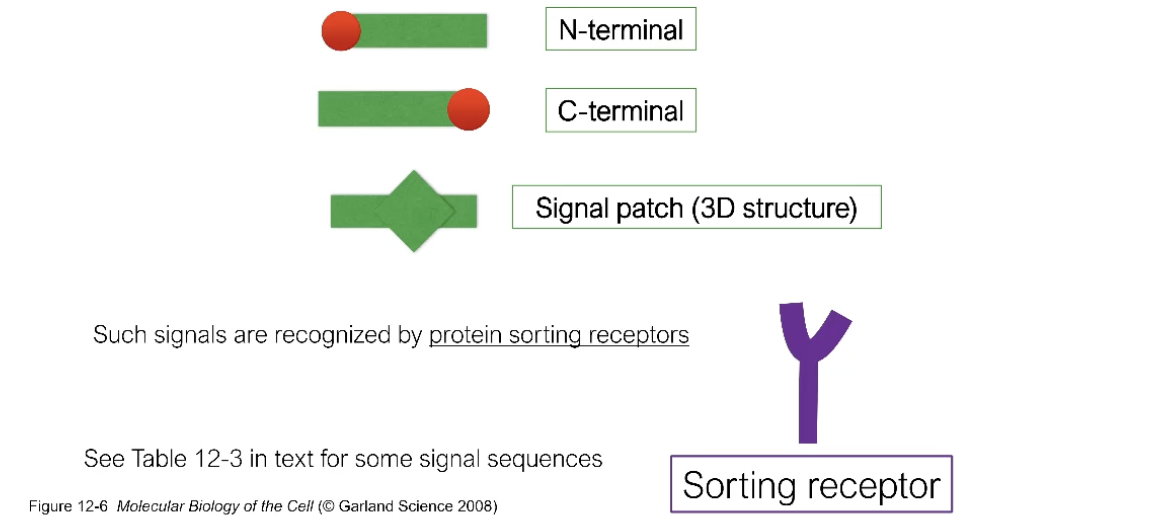
How are proteins targeted to specific organelles?
Through the recognition of signals sequences!
N-terminal, C-terminal, signal patch!
The sorting receptor recognizes these signals! Binds to the signal sequence of the protein and aids in transporting that protein through some transport mehcanism?
What are the three fundamental tansport mechanisms?
Gated transport (cytosol and nucleus via nuclear pore complexes)
Transmembrane transport
Vesicular transport
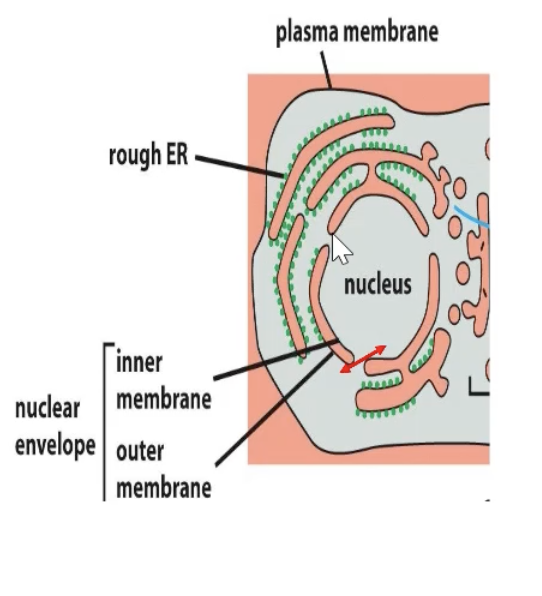
What is gated transport?
Movement between cytosol and nucleus
ER and nucleus are topogically similar (no crossing of lipid bilayer)
Selective transport (active transport from cytosol to nucelus)
Free diffusion of smaller molecules
What is transmembrane transport?
Protein transport between the cytosol and organelles that are topologically different
Through membrane-bound protein translocation
Proteins must be unfolded to snake through the translocator
Cytosol to ER
Cytosol to mitochondria
What is vesicular transport?
Protein transport among topologically equivalent organelles!
Occurs through vesicles
ER to golgi
Golgi to endosomes
Endosomes to lysosomes
Endosomes to plasma membrane
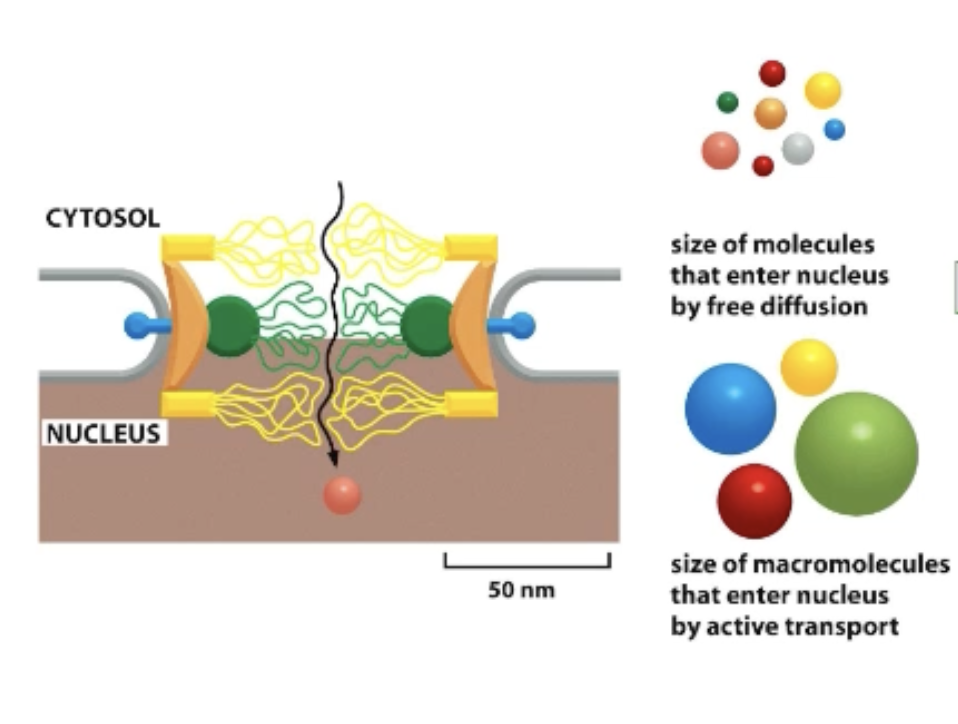
What is the nuclear pore complex?
Nucleoporins line the pore and contain random coils (unstructured regions) that restrict the movement of larger macromolecules!
Free diffusion of up to 9 nm, everything else = signal
What initiates nuclear import?
Nuclear localization signal (NLS) within the cargo must be recognized by nuclear import receptors
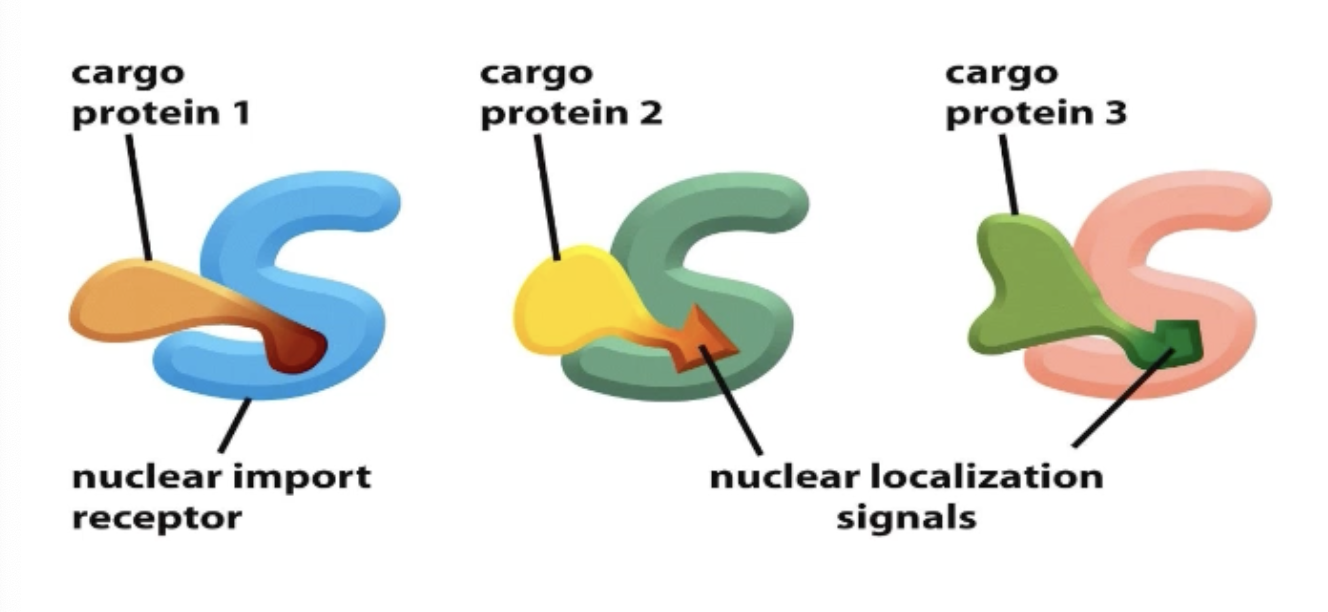
What is cargo?
The thing being exported
What is the NLS?
Any five basic amino acids in a row! The specific sequence does not matter. Just the chemical nature!
Lysine, Arginine, Histidine, Asparagine, etc.
What is the important about the import of nuclear proteins thru pore complex gradient?
Increases order in the cell so it consumes energy through the hydrolysis of GTP (Ran)!
What provides energy from gated transport?
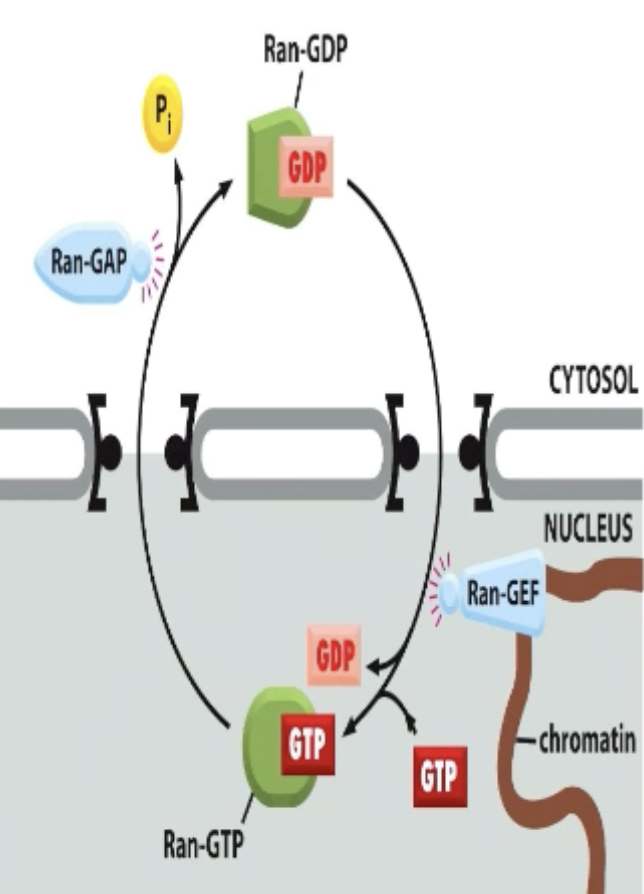
Ran!
Ran exists in both cytosol and nucleus, BUT there is a higher concentration of GTP-Ran in nucleus and GDP-Ran in cytosol!
Why does the Ran concentration gradient exist?
There is more Ran-GEF in nucleus and more Ran-GAP in cytosol.
So more cytsol Ran is GDP bound and more nucleus Ran is GTP.
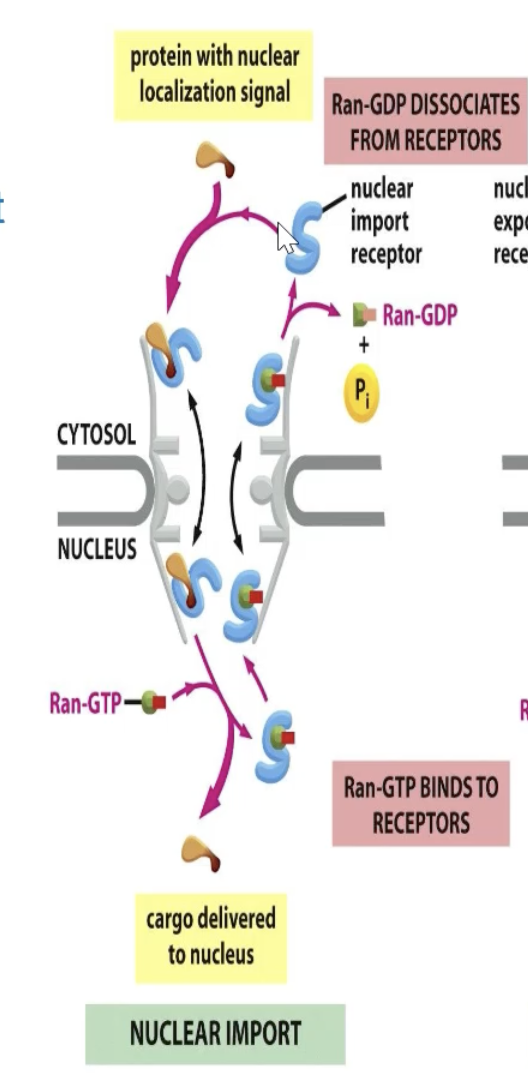
How does nuclear import happen?
To import: Nuclear import receptor binds to cargo if it is NOT bound to Ran
CYTOSOL
GDP bound Ran is inactive and doesn’t bind to receptor
Receptor is open and binds to cargo
NUCLEUS
Interacts and binds with Ran-GTP
Releases cargo
CYTOSOL
Ran receptor complex interacts with Ran-GAP, which hydrolyzes Ran-GTP
GDP bound Ran does not bind
Receptor picks up more cargo.
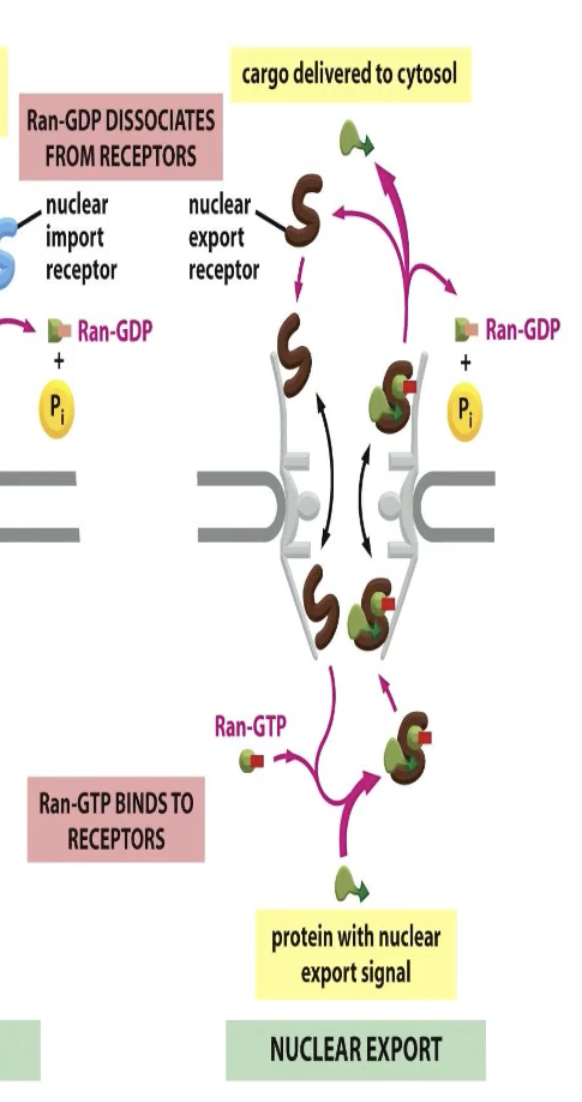
How does nuclear export happen?
Only binds to cargo when it is bound to Ran!
NUCLEUS
Picks up cargo in nucleus where Ran-GTP is high
Both Ran and cargo are bound to receptor!
CYTOSOL
Ran-GAP hydrolyzes GTP
Ran dissociates from receptors
Cargo is released
NUCLEUS
Back into the nucleus and Ran interacts with GEF for GDP to GTP
Receptor moves back into nucelus and binds to Ran
What is the difference between nuclear import and export?
Import: Cargo binds only in the absence of Ran in cytosol (Ran-GDP is the form)
Export: Cargo binds only in the presence of Ran in nucleus (Ran-GTP is the form)
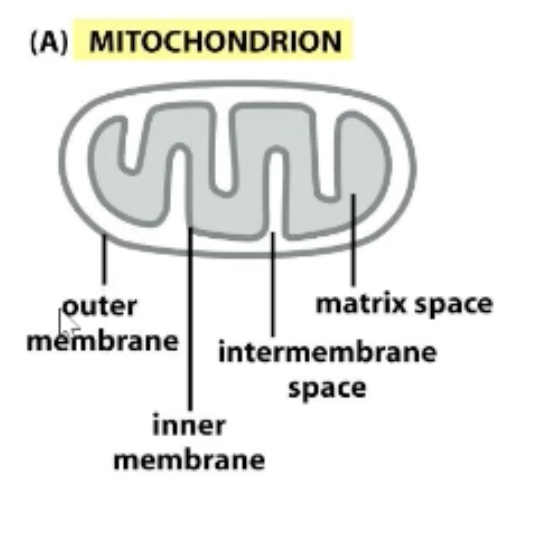
What are the four compartments where proteins can be found in the mitochondrion?
Outer membrane
Inner membrane
Intermembrane space
Matrix space
What is limiting of the mitochondria and chloroplast?
Neither has genome with all the information necessary to code for all proteins.
They rely on the import of their proteins from the cytosol following synthesis.
What is mitochondrial transport?
Mitochondrial proteins are first synthesized as precursor proteins in the cytosol and then translocated into the mitochondria.
Relies on an import signal sequence.
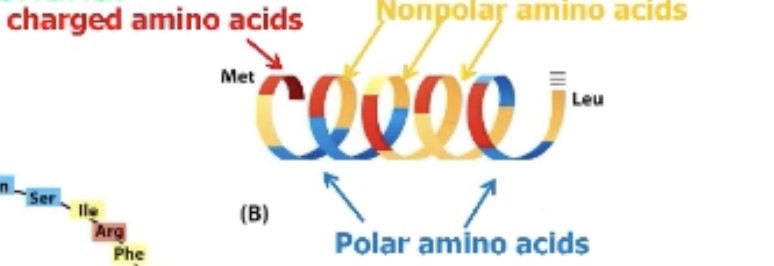
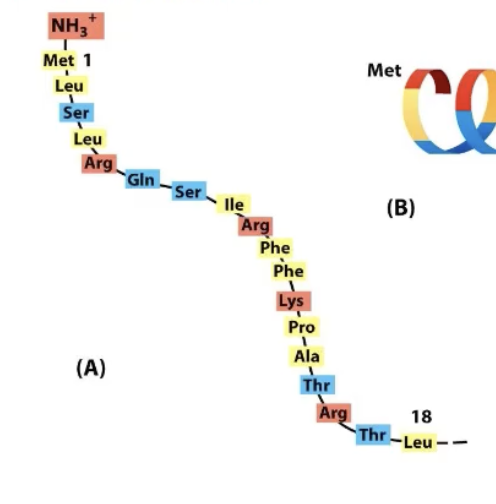
What’s special about the mitochondrial import signal?
Charged amino acid every 3-4 amino acids apart (every helix turn)!
This creates an amphipathic alpha helix, charged amino acids cluster on one face!
All that matters is the chemical nature of amino acids in appropriate locations.
What are the important signal sequences?
Nuclear import (5 basic amino acids)
Mitochondrial import (charged amino acid every; amphipathic)
ER signal sequence (8 or more hydrophobic/nonpolar amino acids in a row)
What are translocators?
Thread the protein across the mitochondrial membrane! Require the protein to be unfolded
TOM (translocase of outer mitochondrial membrane)
TIM (translocase of inner mitochondrial membrane) complexes
TIM23 and TIM22
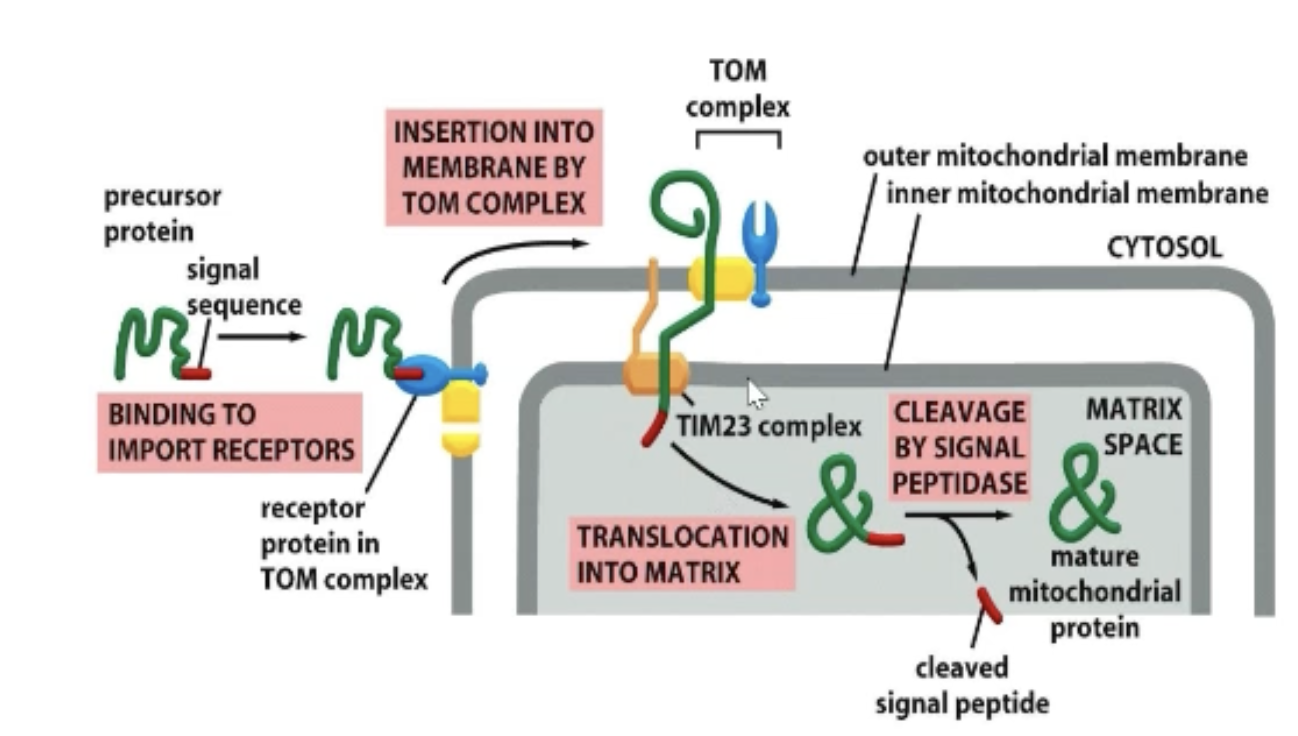
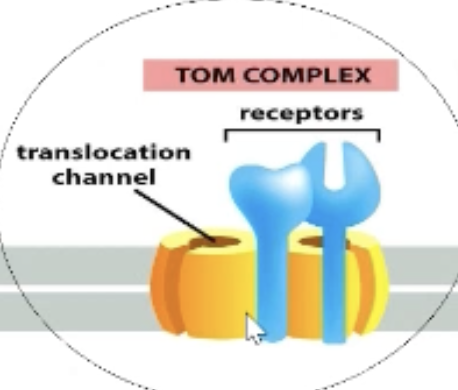
What is the TOM complex?
Contains the receptor for mitochondrial import signal.
Passes protein to translocation channel (passes outer lipid bilayer).
All proteins interact with TOM complex (gate-keeper into mitochondria).
What is the SAM complex?
Sorting and Assembly Machinery (SAM)
Transmembrane proteins (with beta barrel structure) are transferred to the SAM complex. Help proteins insert and fold properly.
NOT TESTED**
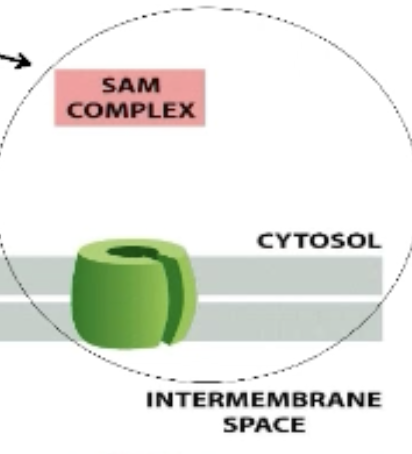
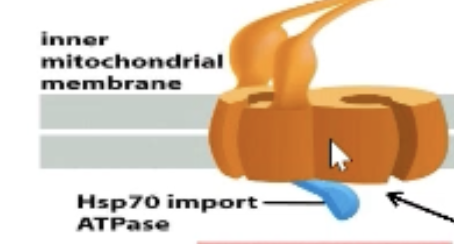
What is the TIM complex?
TIM22 complex only in inner mitochondrial membrane!
TIM23 complex spans BOTH membranes
Can interact with TOM complex
Translocation channels on inner mitochondrial membrane
Heat Shock Protein (Hsp)70 import ATPase
When do you need TIM v. TOM?
Soluble to INTERMEMBRANE space → TOM
Soluble to MATRIX space or membrane proteins to INNER MITOCHONDRIAL MEMBRANE → TIM + TOM
What does Hsp70 import ATPase do?
Harnessed by TIM to pull protein across TIM complex into mitochondrial matrix.
Most of them are chaperones for prevention of protein folding.
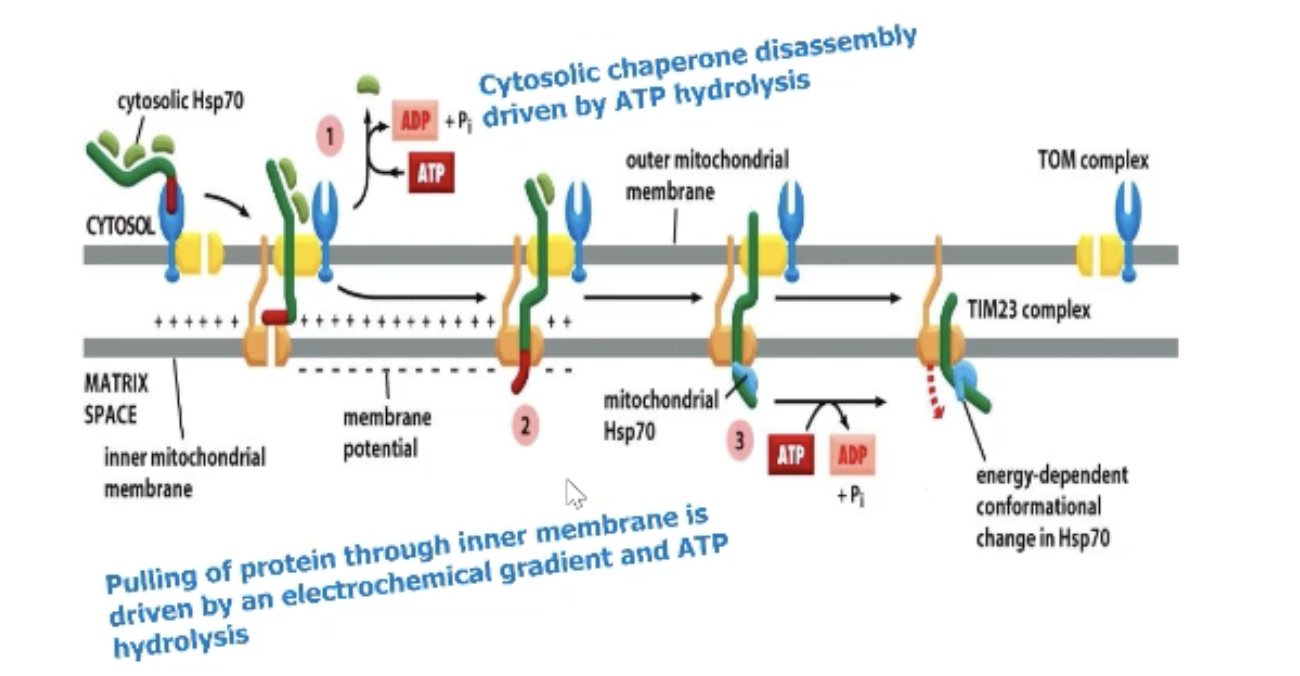
An exception to GTP for regulation?
Transporting proteins across mitochondria is ATP dependent with Hsp70.
How are proteins prevented from folding up?
They are bound by chaperones in the cytosol to hold in unfolded state!
OR
If it does fold up first, chaperone proteins can hydrolyze ATP to unfold the protein
MOST COMMON CHAPERONE: Hsp70

Where does energy come from for mitochondrial protein import?
Membrane potential AND hydrolysis of ATP
What is transport of proteins across ER (co-translational translocation)?
Proteins imported into ER as they are being synthesized (don’t have to worry about unfolding)!
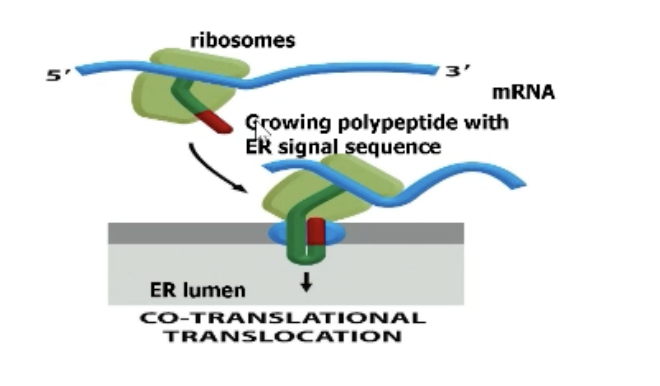
What types of proteins undergo co-translational transolocation?
Any soluble proteins found in lumen of non-nucelar, non-mitochondria, non-chloroplast organelles!
ER, Golgi, lysosomes, etc.
Anything that needs to be secreted out of the cell (ex. hormones)
Transmembrane proteins that a need to be in the membrane of those organelles.
What is the ER signal sequence?
Not sequence specific and hydrophobic (8 or more hydrophobic/nonpolar amino acids in a row)
Most are N-terminal, but not all.
MUST BE RECOGNIZED BY THE SRP (signal recognition particle)
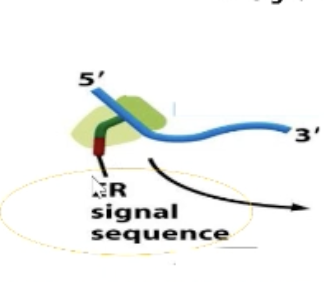
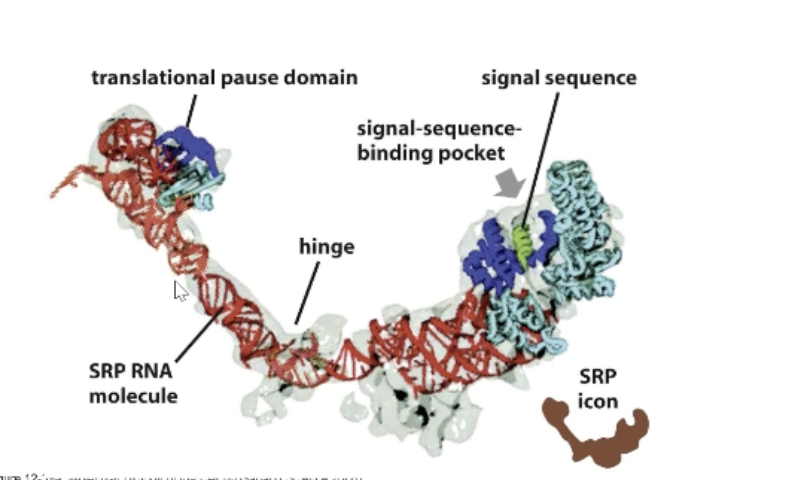
What is the SRP?
Signal recognition particle that recognizes ER signal sequence! Interacts with SRP receptor.
Composed of proteins and RNA.
Protein Component (signal sequence binding pocket): Interacts with new protein
RNA Component (translational pause domain): Prevents elongation factors from binding
Would you consider the SRP to be a ribozyme?
No!
If it were a ribozyme it would catalyze a chemical reaction!
An RNA component does not necessary mean its an enzyme.
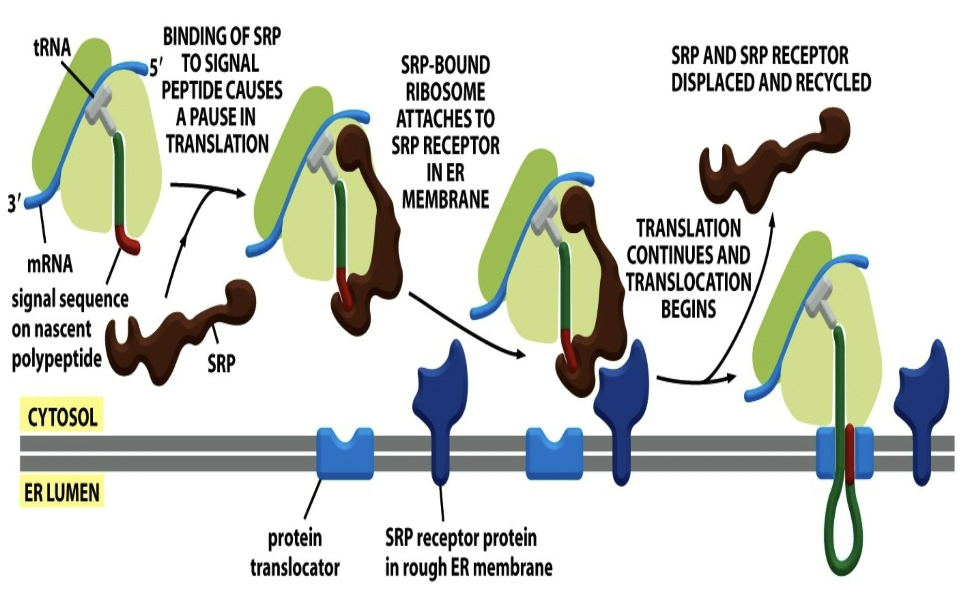
How does ER translocation steps (steps)?
1) Binding to SRP from signal sequence.
2) Whole thing binds to SRP receptor in ER membrane
3) Translation continues and translocation beings
4) SRP receptor and SRP release and are recycled; protein is pushed into ER
How does ER translocation work for transmembrane proteins?
Membrane must be stuck in membrane via two hydrophobic regions.
There is a hydrophobic stop transfer sequence that anchors the protein the membrane! Replaces the start transfer sequence.
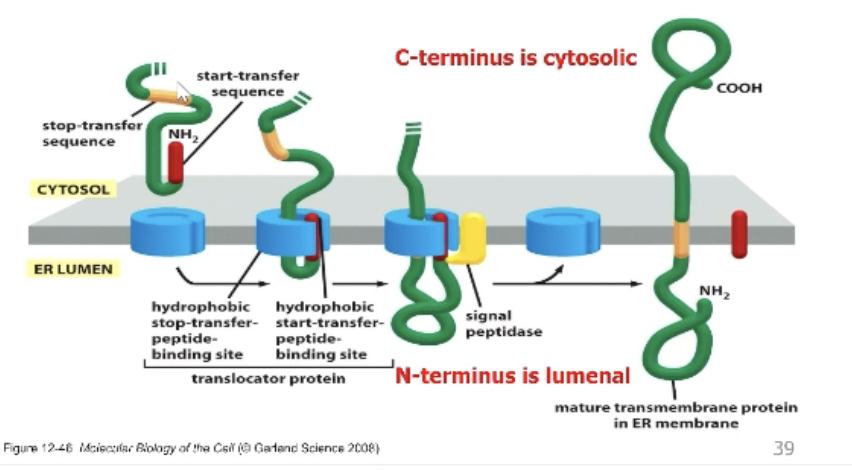
What’s key to protein orientation of transmembrane ER protein?
Location of charged amino acid:
The + charge will always face the cytosol!
# of transmembrane regions!
Odd # = opposite sides of membrane
Even # = same sides of membrane
Either N terminus or C terminus can be pushed into the lumen!
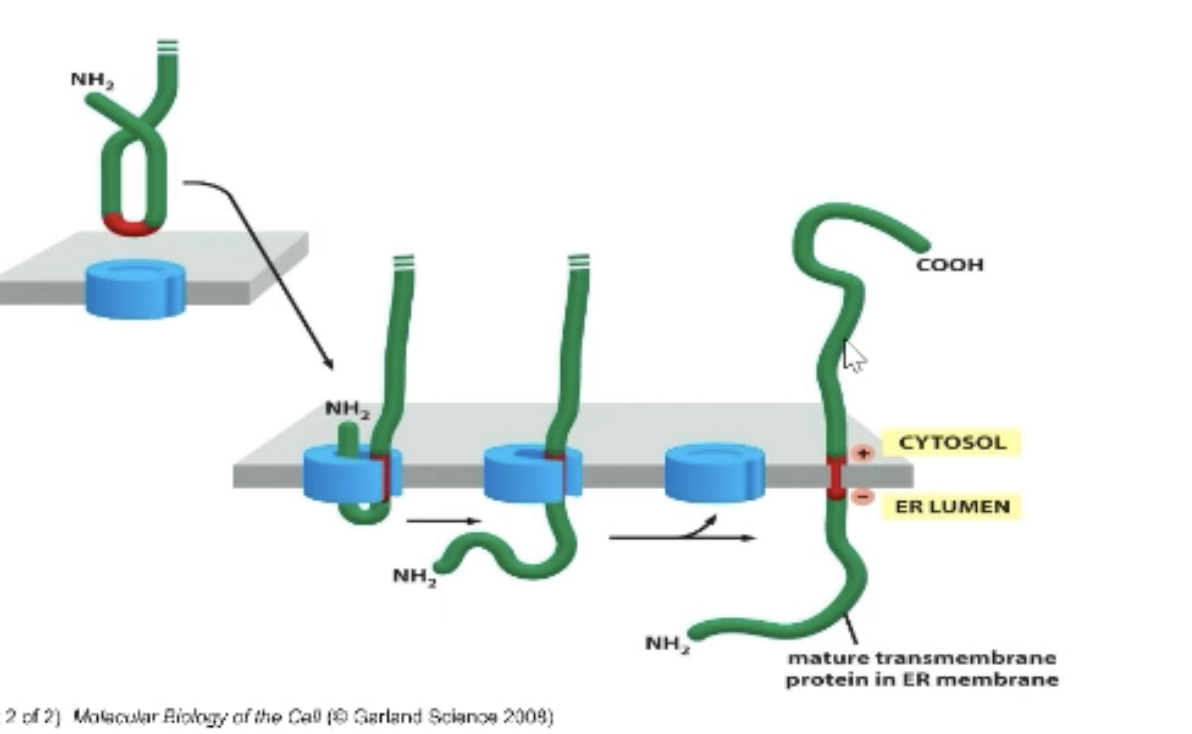
How is orientation determined with multi transmembrane regions (trick)!
Hydropathy plot tells us how many transmembrane regions we have! Determine how many (even or odd number)!
Determine + and - for first transmembrane region and which is N and C-terminal.
+ is always towards the cytosol!
Use information to determine orientation.
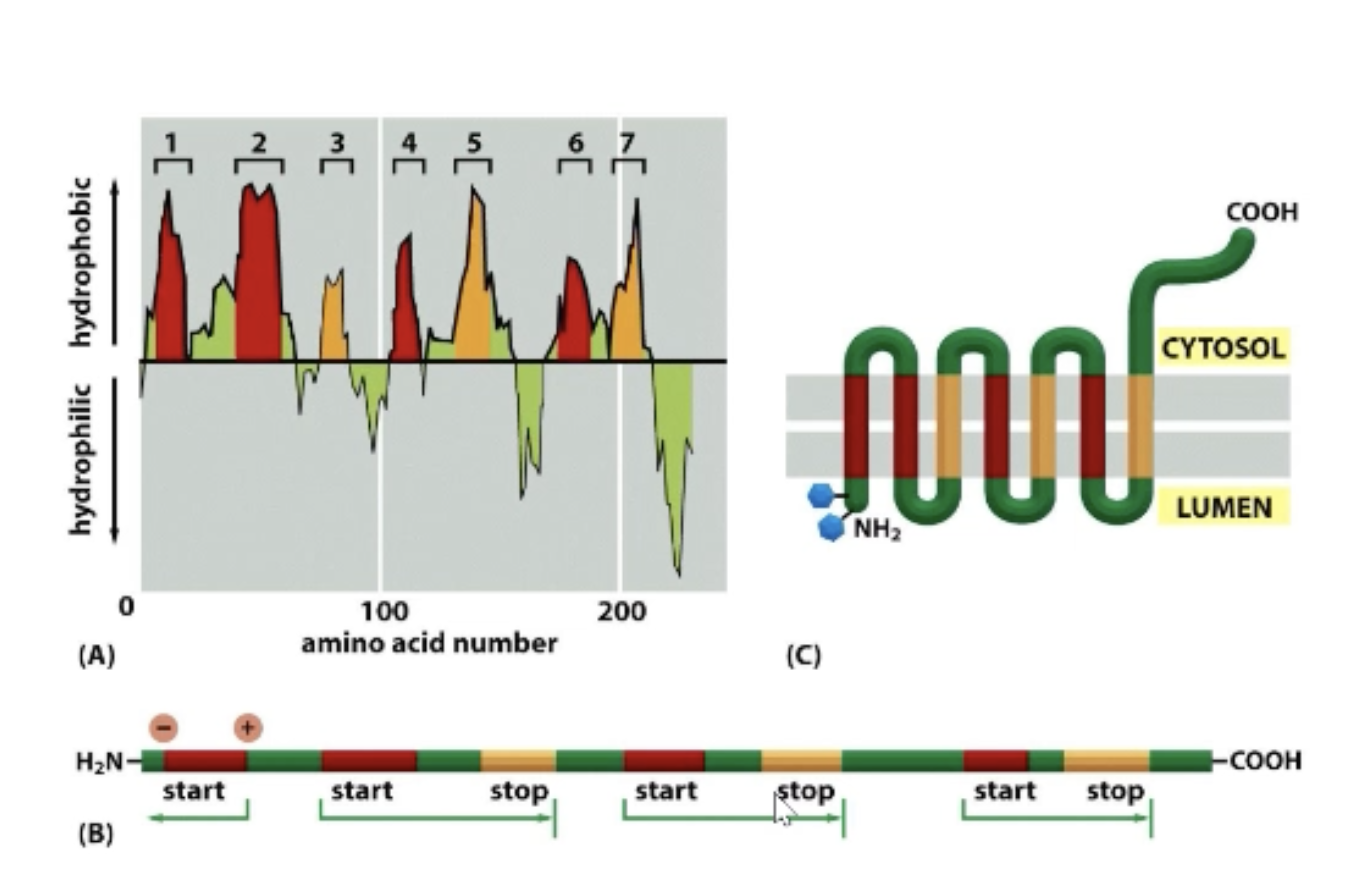
What’s the purpose of a transmembrane region?
Reverse the orientation of protein!