Chemistry 必記 ( j2 ver)
1/158
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
159 Terms
Is halogenoalkane polar or non polar?
polar due to polar carbon-halogen bonds
Compare the boiling point of halogenoalkane and alkane of similar Mr
boiling point of halogenoalkane is higher than alkane of similar Mr
a higher amount of energy is needed to overcome the stronger permanent dipole permanent dipole between the polar halogenoalkane molecules as compared to the weaker instantaneous dipole induced dipole attractions between non polar molecules
State the trend of boiling point and increasing halogen size
for given alkyl group, boiling point increases with larger halogen size
size of electron cloud for : CH3I > CH3Br > CH3Cl
extent of distortion of electron cloud for : CH3I > CH3Br > CH3Cl
extent of intermolecular instantaneous dipole-induced dipole attractions: CH3I > CH3Br > CH3Cl
amount of energy required to overcome the intermolecular instantaneous dipole-induced dipole attractions : CH3I > CH3Br > CH3Cl
Halogens are ___ in organic solvent
soluble
Halogens are ___ in water
insoluble
Why is halogenoalkane soluble in organic solvents?
energy released by the formation of instantaneous dipole-induced dipole attractions between halogenoalkane and organic solvent molecules is sufficient to overcome the instantaneous dipole-induced dipole attractions between organic solvent molecules, and between halogenoalkane molecules, hence they are soluble in organic solvents
Why is halogenoalkane soluble in water?
energy released by the formation of weak instantaneous dipole-induced dipole attractions between halogenoalkane and water molecules is insufficient to overcome the strong hydrogen bonding between water molecules and instantaneous dipole-induced dipole attractions between halogenoalkane molecules, hence they are insoluble in water
Sn2 nucleophillic substition, mechanism can be used for ___
primary halogenoalkane
Sn1, nucleophillic substition, mechanism for ___
tertiary halogenoalkane
Sn2 mechanism is a ___
single step reaction, overall secondary kinetics
Sn1 mechanism is a ___
2-step reaction
reaction follows first order kinetics
Draw the energy profile diagram of the SN1 hydrolysis reaction
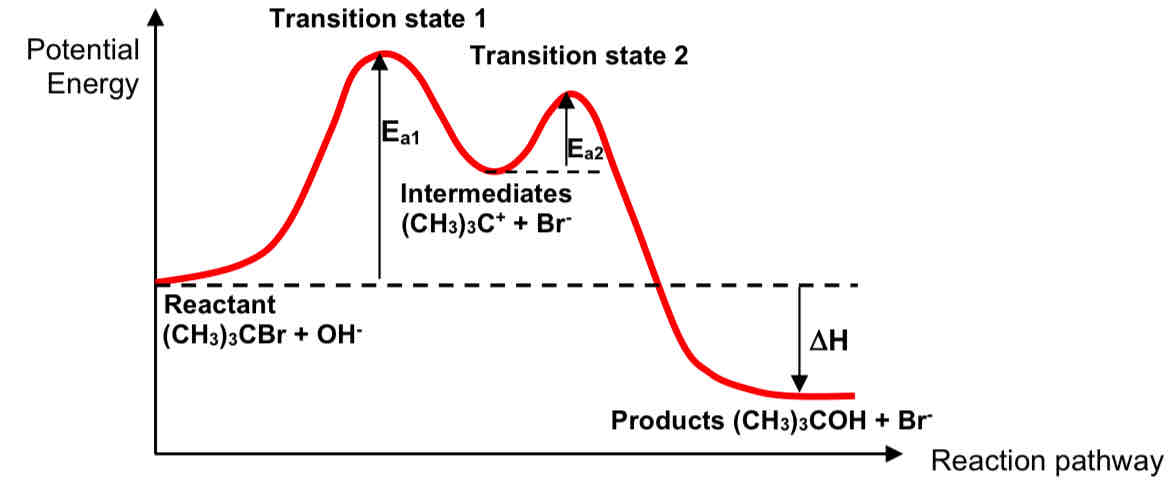
Explain why a racemic mixture is obtained when the halogenoalkane is chiral
the nucleophile attacks the sp2 hybridised, trigonal planar carbocation intermediate from either the top or bottom of the plane with equal probability.
this leads to the formation of equal amounts of each (+) and (-) enantiomers resulting in the formation of a racemic mixture
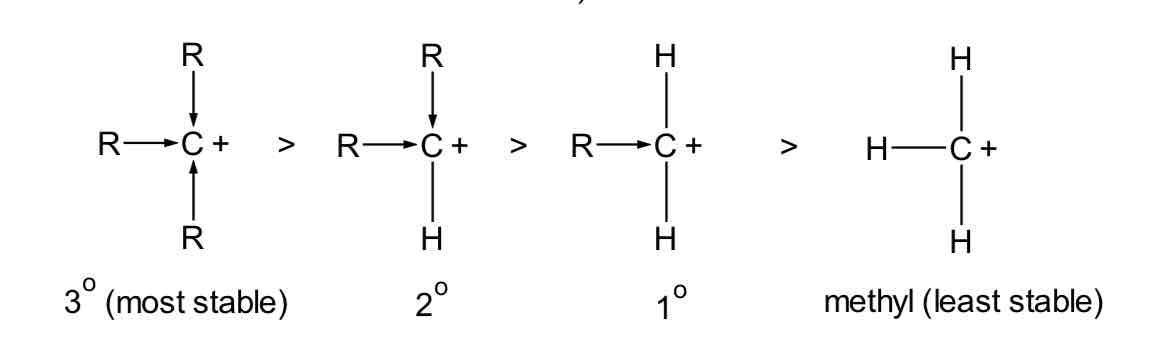
Explain why a tertiary RX undergoes SN1 but a primary RX does not
relative rate of reaction of SN1 reaction: 3°> 2°> 1°> methyl
this is due to the stability of carbocation formed (more stable carbocation formed would lead to faster reaction)
Sn1 forms __
carbocation intermediate during the slow step of the reaction
Sn2 forms __
pentavalent transition state during the reaction
When is Sn1 more favourable?
mechanism is favoured when the carbocation formed is more stable
When is Sn2 more favourable?
mechanism is favoured when there is less steric hindrance on the organic reactant molecule for nucleophilic attack
What is observed when R Cl reacts with NaOH?
slight cloudiness after 1 hour
white precipitate (AgCl) is observed
What is observed when R Br reacts with NaOH?
cloudiness appears after a few minutes
cream precipitate (AgBr) is observed
What is observed when R I reacts with NaOH?
thick precipitate occur in a minute
pale yellow precipitate (AgI) is observed
Describe a chemical test to differentiate halogenoalkanes
heat compound with NaOH(aq) followed by dilute HNO3
add AgNO3
What is the application of nucleophilic substitution reactions with CN (nucleophile)?
increase the no. of C atoms in a chain (1C for each substitution of X with CN), (step-up reaction)
resulting nitrile product, RCN can be hydrolysed to form carboxylic acids or reduced to form amines
State an exception due to steroid hindrance
1-chloro-2,2-dimethylpropane, (CH3)3CCH2Cl, despite being a primary halide, it favours the SN1 mechanism instead of SN2 because of steric hindrance of the bulky −C(CH3)3 group
State the exception due to electronic consideration
(chloromethyl)benzene, C6H5CH2Cl, being a primary halide, favours substitution by the SN1 mechanism due to resonance stabilisation of the carbocation intermediate by dispersal of positive charge due to delocalisation of pi electrons.
Explain why primary RX undergoes SN2 while tertiary RX does not
due to steric hinderance, it is easier fro nucleophile to attack C atom if there are less bulky R groups around the C atom
What is the purpose of nucleophilllic substitution reactions with CN- (nucleophille)
to increase the no. of C atoms in a chain (1C for each substitution of X with CN), i.e. step-up reaction
the resulting nitrile product, RCN can be hydrolysed to form carboxylic acids or reduced
to form amines
General equations for Reactions of Nitriles
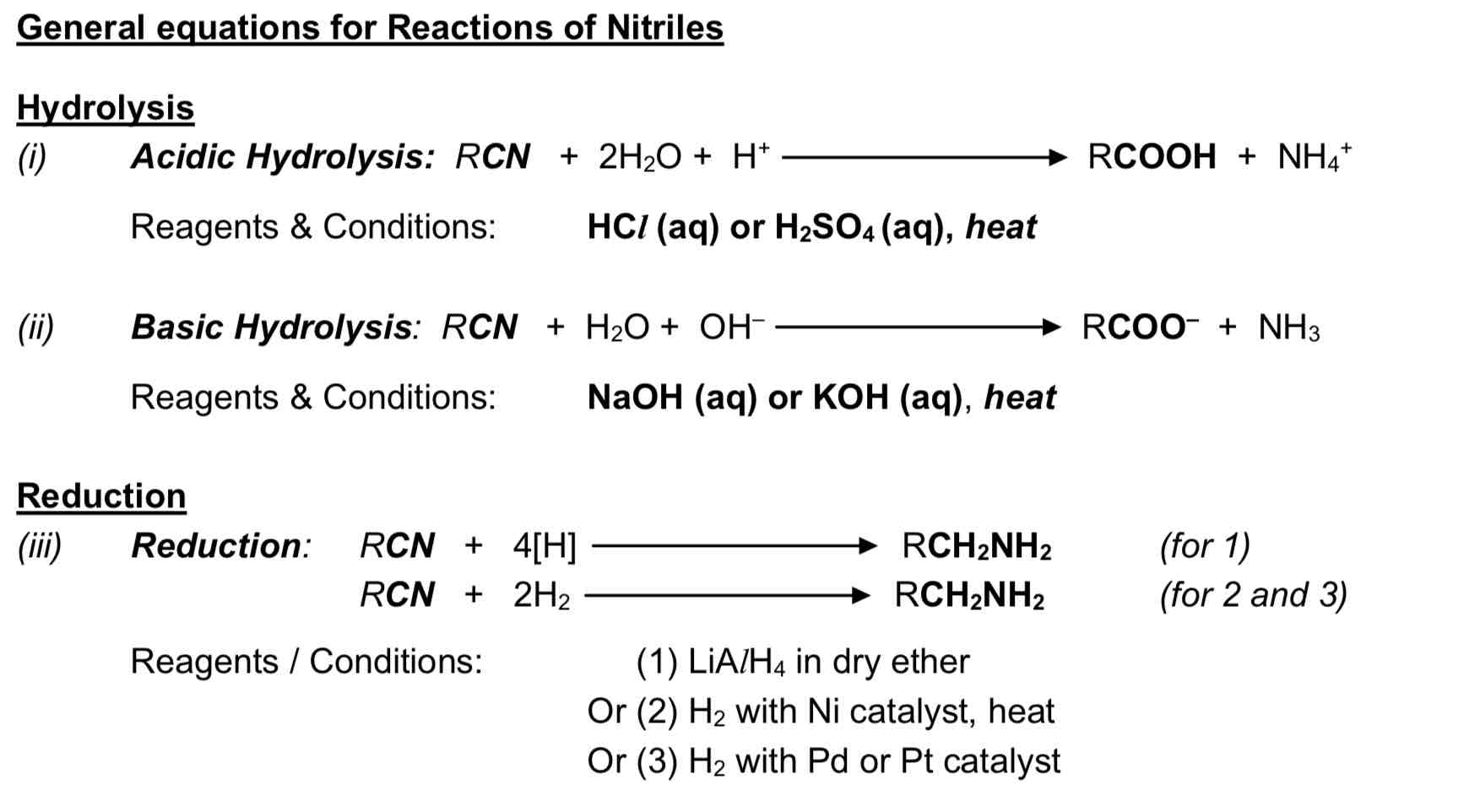
State the reagent and condition needed to form amine
reagent & condition: excess concentrated ammonia in ethanol, heat in sealed tube
type of reaction: nucleophillic substitution
Account for the difference in reactivity of halogenarenes and halogenoalkanes
in halogenoarenes, the C-X bond is shorter and stronger than those in halogenoalkanes.
the lone pair of electrons on the halogen atom is delocalised into the benzene ring.
this strengthens the carbon halogen (CX) in halogenoarenes due to presence of partial double bond character
in addition, the pi-electron cloud of the benzene ring will repel the lone pair of electrons of the incoming nucleophile, hence rendering attack of the nucleophile difficult
Why do halogenoalkenes not undergo nucleophillic substitution readily
lone pair on the halogen atom is delocalised with the adjacent C=C.
this strengthens the carbon-halogen (C-X) bond in halogenoalkenes due to presence of partial double bond character, hence nucleophilic substitution does not occur under normal conditions
Relative Ease of Hydrolysis of Alkyl and Aryl Chlorides
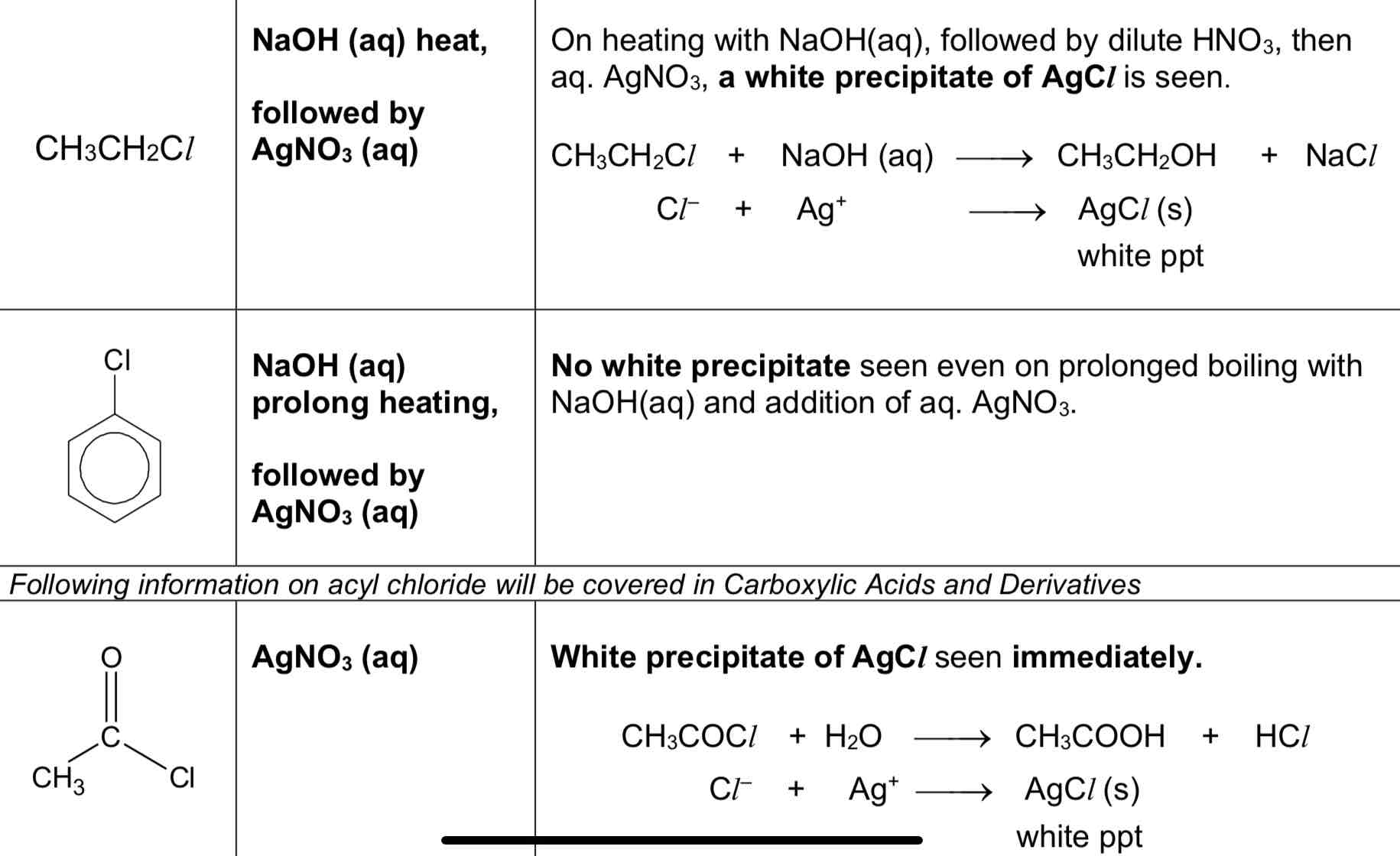
Explain the rate of nucleophilic substitution of alkyl chloride
there is only one electronegative chlorine atom bonded to the alkyl C atom
hence, the alkyl C atom is less electron deficient
less susceptible to nucleophilic substitution compared to the acyl chloride
Explain the rate of nucleophilic substitution of halogenoarenes
lone pair of electrons on the chlorine atom is delocalised into the benzene ring
this strengthens the carbon-halogen (C–X) bond in halogenoarenes due to presence of partial double bond character
nucleophilic substitution does not occur under normal conditions
Explain the rate of nucleophilic substitution of acyl chloride
the carbonyl C atom is bonded to two electronegative atoms O and Cl
this makes the carbonyl C atom highly electron-deficient
hence, the highly electron deficient carbonyl C atom is very susceptible to nucleophilic substitution which occurs readily
What are some uses of fluroalkanes and chlorofluroalkenes
refrigerant
CFCs such as CCl2F2 are commonly used as they are easily liquefied due to low boiling points
aerosol propellant
CFCs are inert, non-toxic and volatile hence an ideal choice
fire extinguisher
CBr2ClF being fully halogenated is non-flammable, volatile and dense hence an ideal choice
Why are fluroalkanes and chlorofluroalkenes generally stable
due to the strong carbon-fluorine (C–F) bond
the combination of C–F and C–Cl binds in a molecule lead to properties of great stability and non-flammability
What happens during the depletion of the ozone layer
it results in UV radition causing skin cancer, damages to vegetation and accelerates greenhouse effect which affects climate change
State the physical properties of alcohol
high boiling point
solubility in water
amphipathic
generally high boiling point
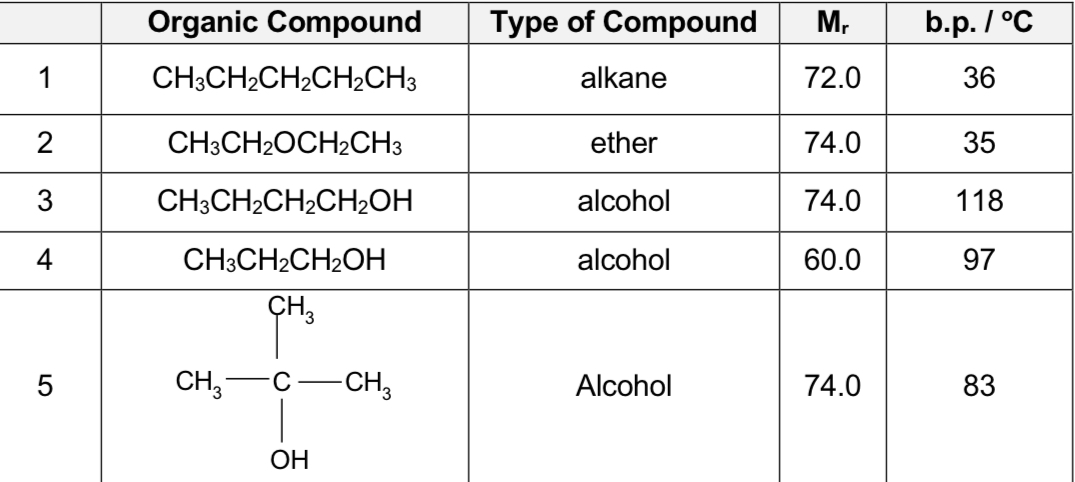
Compare carbon compounds 1,2,3
alcohols have much higher b.p. than hydrocarbons and other organic compounds of comparable size of electron cloud due to the stronger intermolecular hydrogen bonding which require more energy to overcome
thus 3 has the highest boiling point
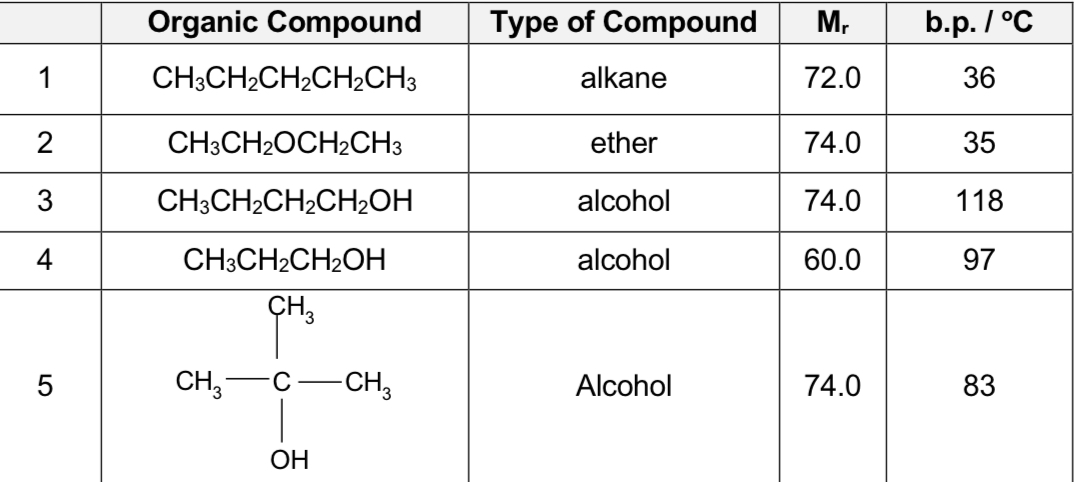
Compare carbon compound 3,4
Boiling point increases with increasing size of molecules.
larger electron cloud size results in a greater extent of distortion of the electron cloud
resulting in more extensive intermolecular instantaneous dipole-induced dipole attractions
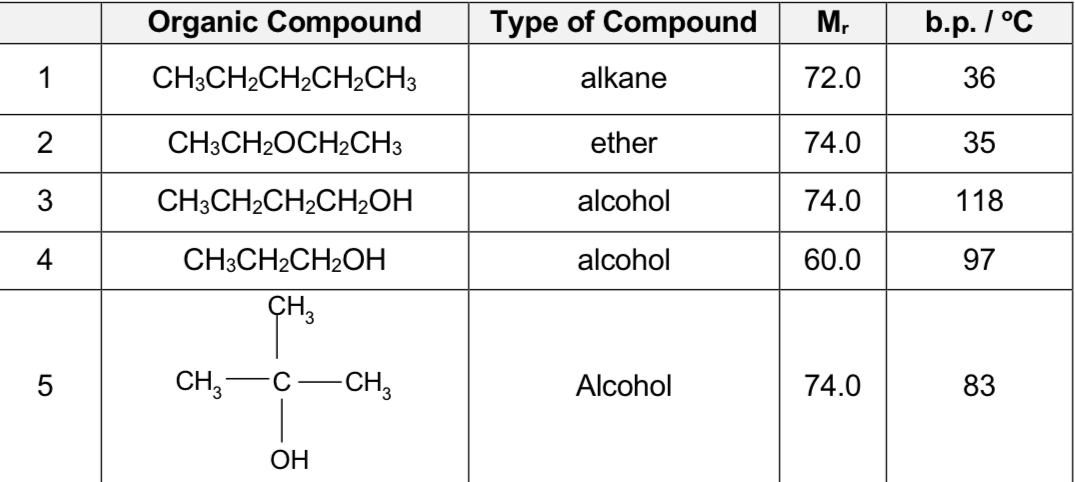
Compare carbon compound 3,5
3 is straight chan while 5 is breached chain
straight-chain isomers have a greater surface area of contact hence giving rise to more extensive intermolecular instantaneous dipole-induced dipole attractions, this straight chain have higher boiling point than branched chains
What bonds can be formed between alcohols and why?
instantaneous dipole-induced dipole attractions and hydrogen bonds can be formed due to alcohol having
a non-polar alkyl group and a polar –OH group
when number of carbon increase, alcohol’s solubility in water __
decreases
aldehydes (RCHO) undergo reduction to form __ alcohols
primary
ketones (RCOR’) undergo reduction to form __ alcohols.
secondary
Why are alcohols with small number of carbons completely miscible in water?
energy released by the formation of hydrogen bonds between alcohol and H2O molecules (solute-solvent interactions) is
sufficient to overcome the intermolecular hydrogen bonds between water molecules, (solvent-solvent interactions) and hydrogen bonds between alcohol molecules. (solute-solute interactions)
Why are alcohols generally very weak acids?
electron-donating alkyl (R) group intensifies the negative charge on the conjugate base (alkoxide ion, RO–), hence destabilising the conjugate base
Why does more carbon affect the solubility of alcohol?
larger non-polar R group makes the alcohol molecule more hydrophobic in nature
as main interaction between larger alcohol molecules and H2O molecules becomes instantaneous dipole–induced dipole attraction instead
energy released from the instantaneous dipole–induced dipole attraction between alcohol and H2O molecules (solute-solvent interactions) is insufficient to overcome the intermolecular hydrogen bonds between water molecules (solvent-solvent interactions)
and the hydrogen bonds (and instantaneous dipole–induced dipole attraction) between alcohol molecules
State the laboratory preparation for alcohol
reagent & conditions: cold concentrated H2SO4 followed by heating with water
type of reaction: electrophilic addition
State the industrial preparation for alcohol
reagent & conditions: steam or H2O (g), high temperature and pressure, and concentrated H3PO4 as catalyst
type of reaction: electrophilic addition
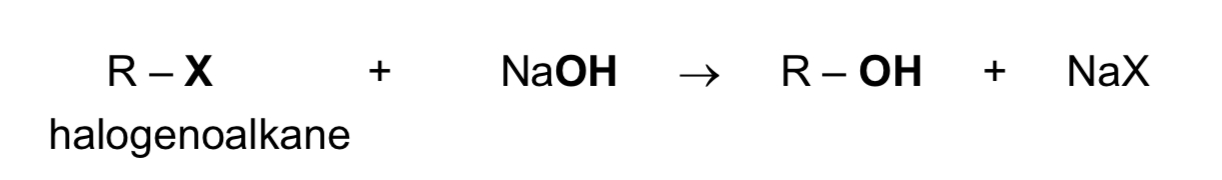
State the nucleophilic substitution of halogenoalkanes
reagent & conditions: NaOH(aq) or KOH(aq), heat
type of reaction: nucleophilic substitution
What are the reagents and conditions for reduction?
LiAlH4 in dry ether
H2, Pt or Pd
H2, Ni, high temperature & pressure
NaBH4 in ethanol (alcohol only)
What are the reactions of alcohols?
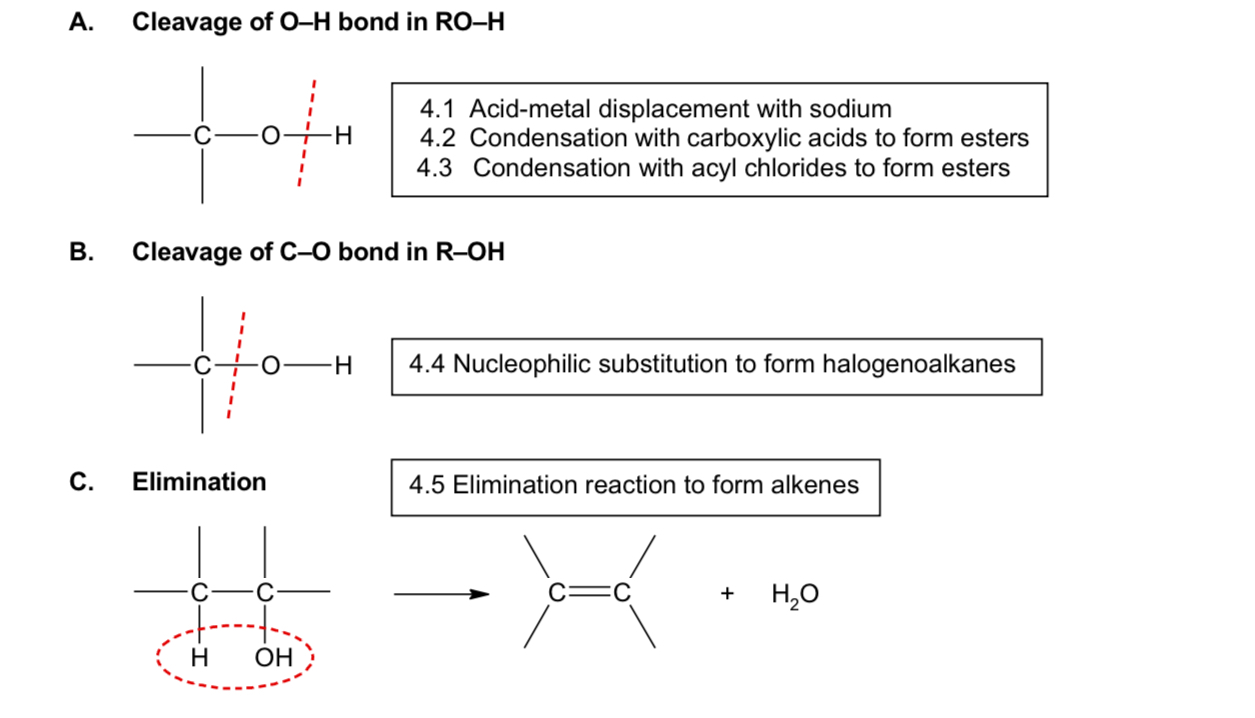
cleavage of C-O bond in R-OH
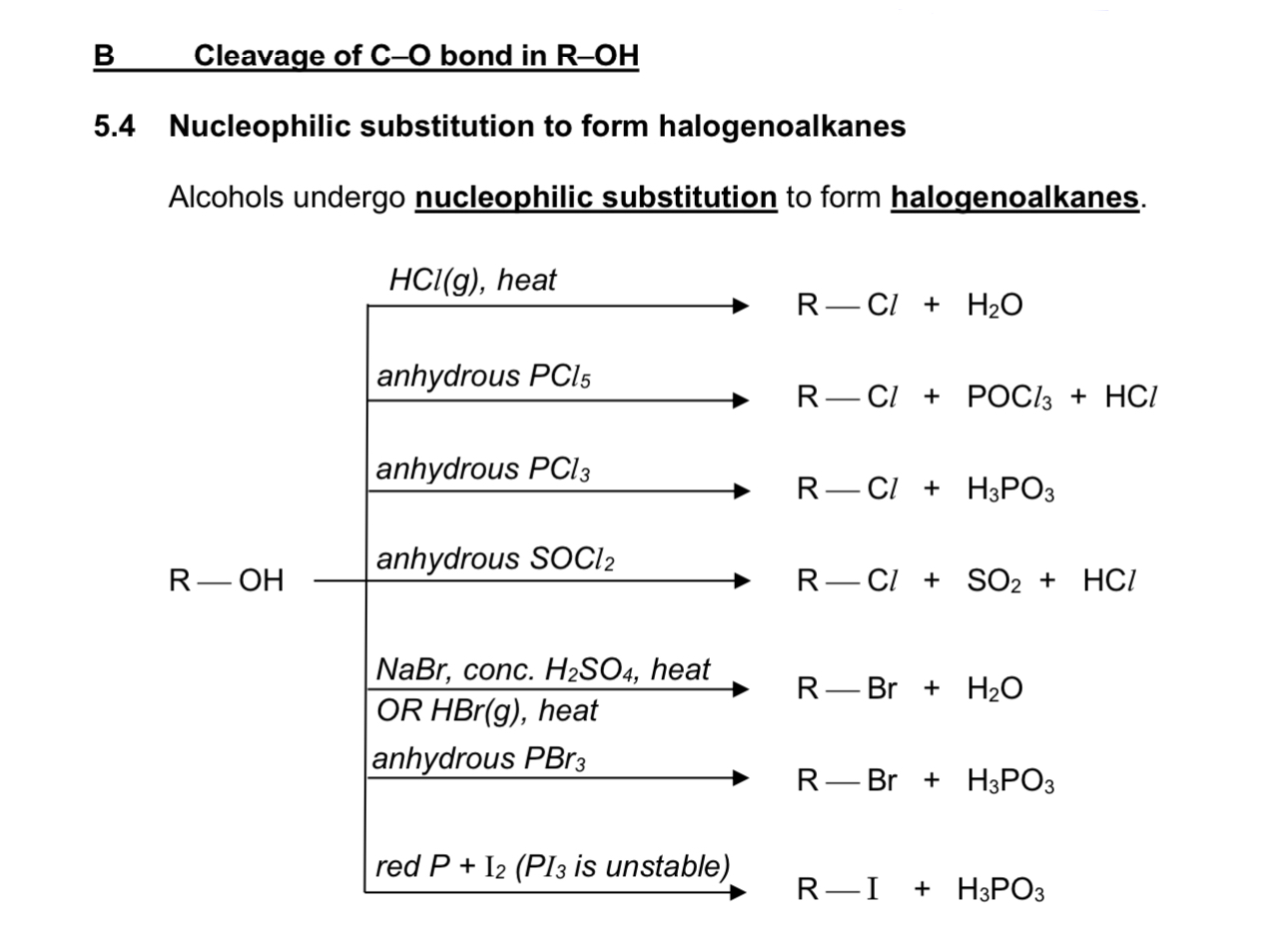

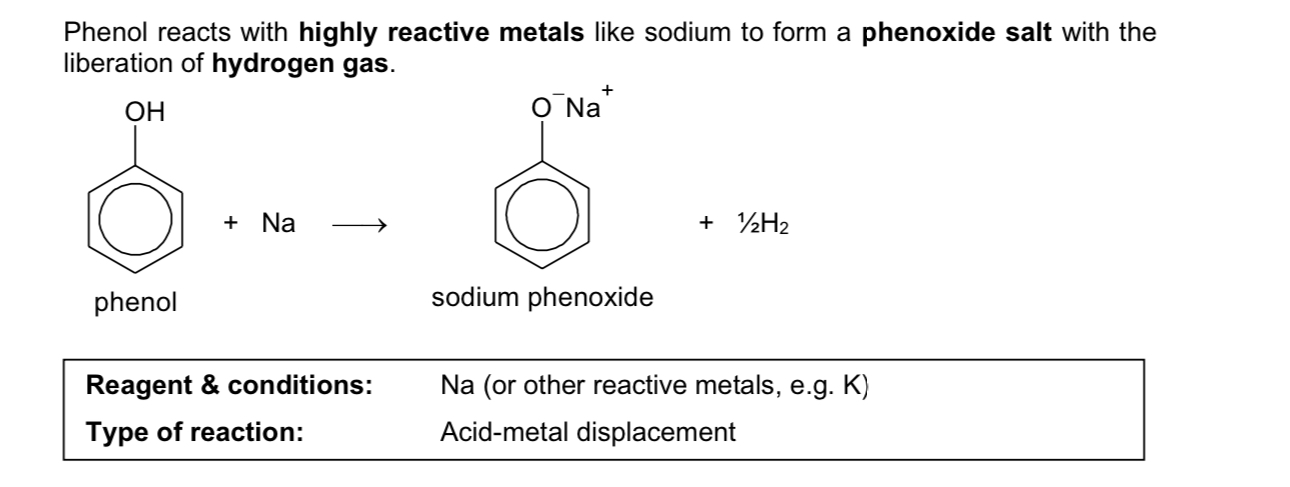
Describe acid metal displacement reaction
reagent & conditions: Na/K (or other reactive metals)
type of reaction: acid-metal displacement
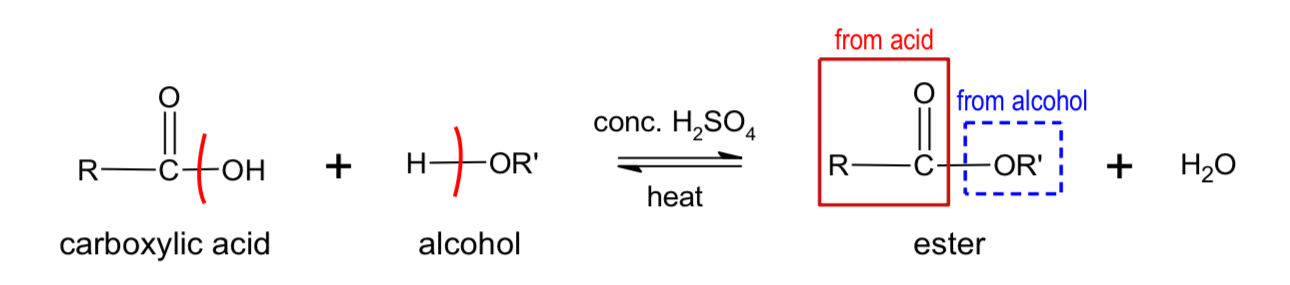
What do alcohols form when reacting with carboxylic acid?
ester

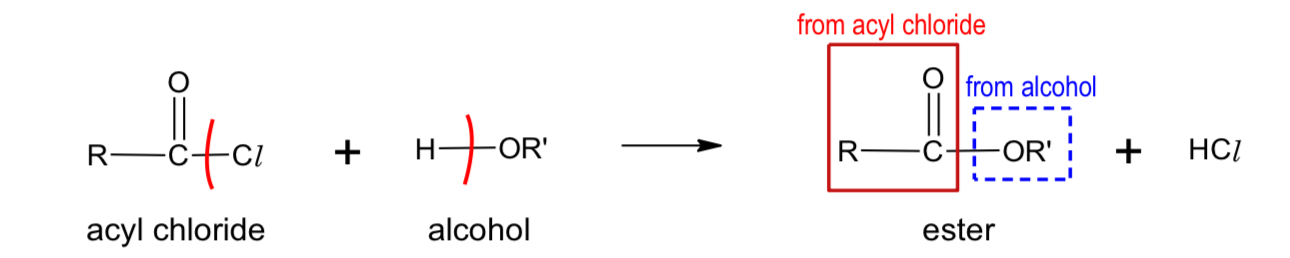
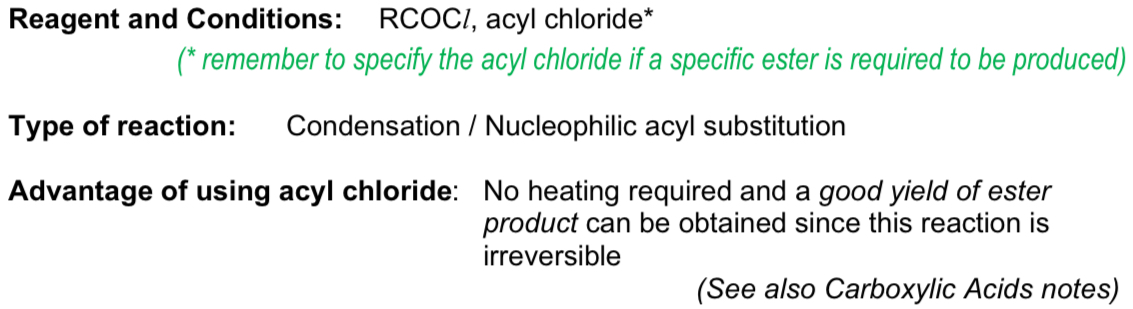
What do alcohols form when reacting with acyl acid?
ester
Why can’t tertiary alcohols be oxidised?
oxidation of an alcohol involves the cleavage of the α-C-H bond, which is absent in tertiary alcohols
special cases in oxidation
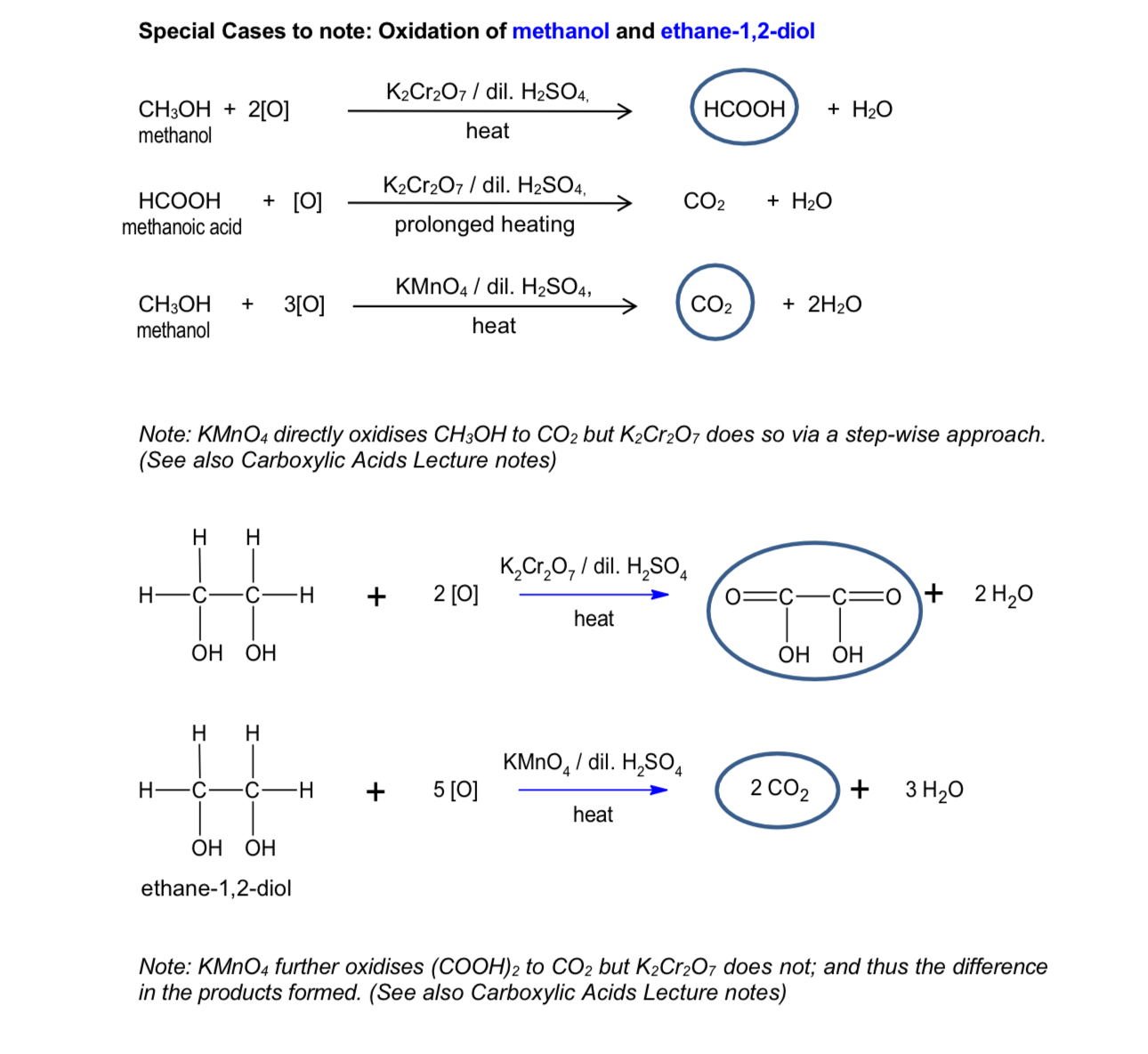
oxidation of primary alcohols
mild oxidation of primary alcohols
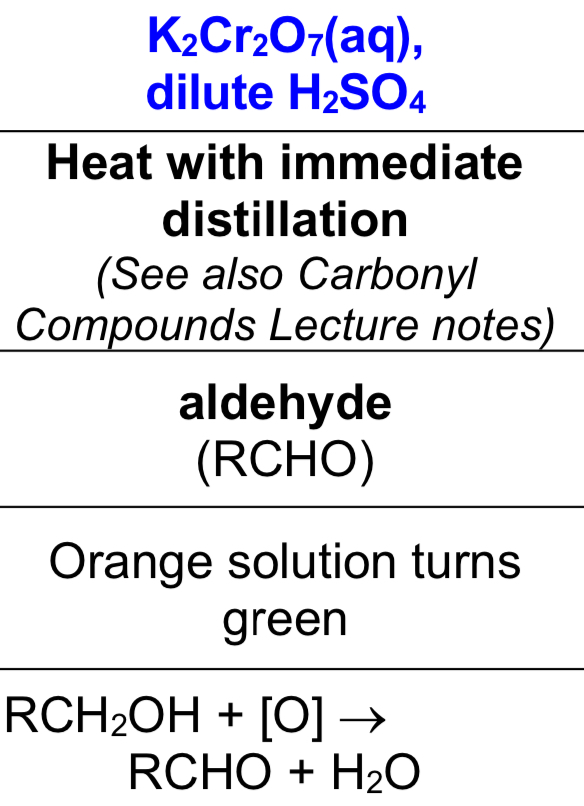
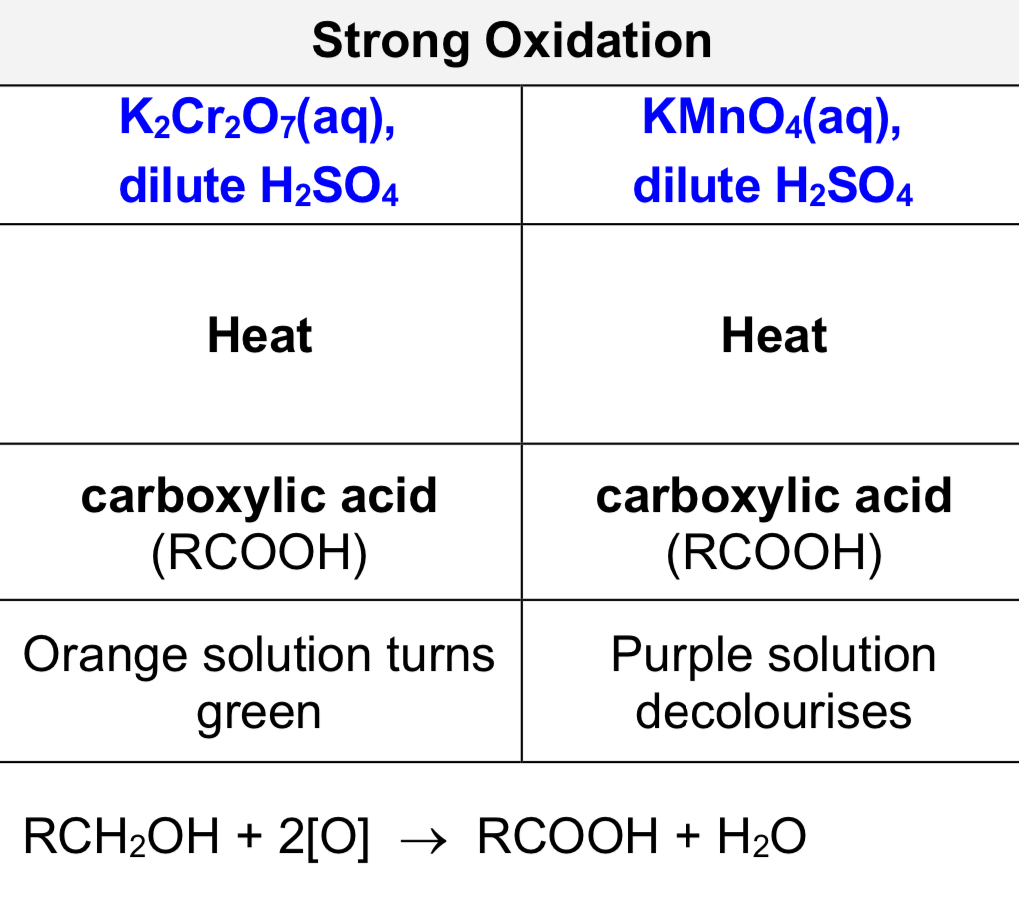
strong oxidation of primary alcohols
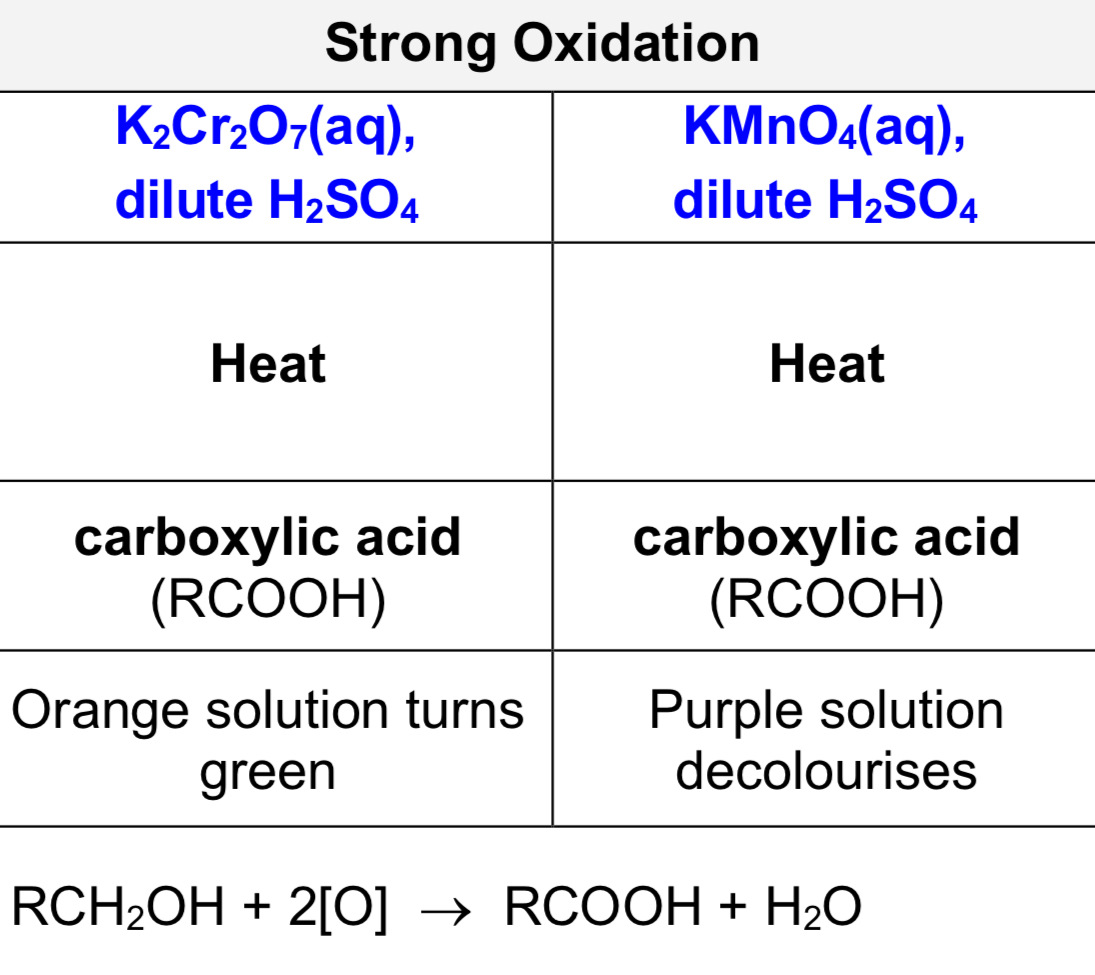
oxidation of secondary alcohols
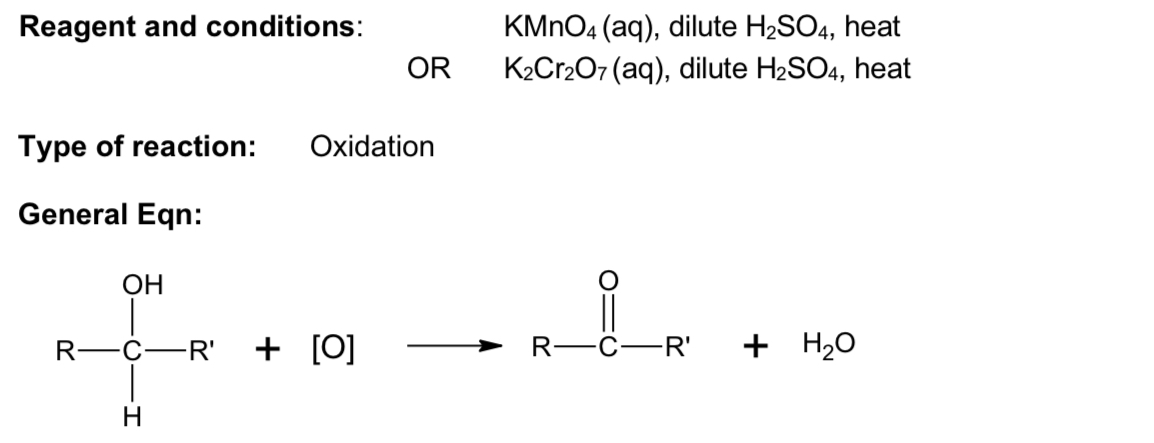
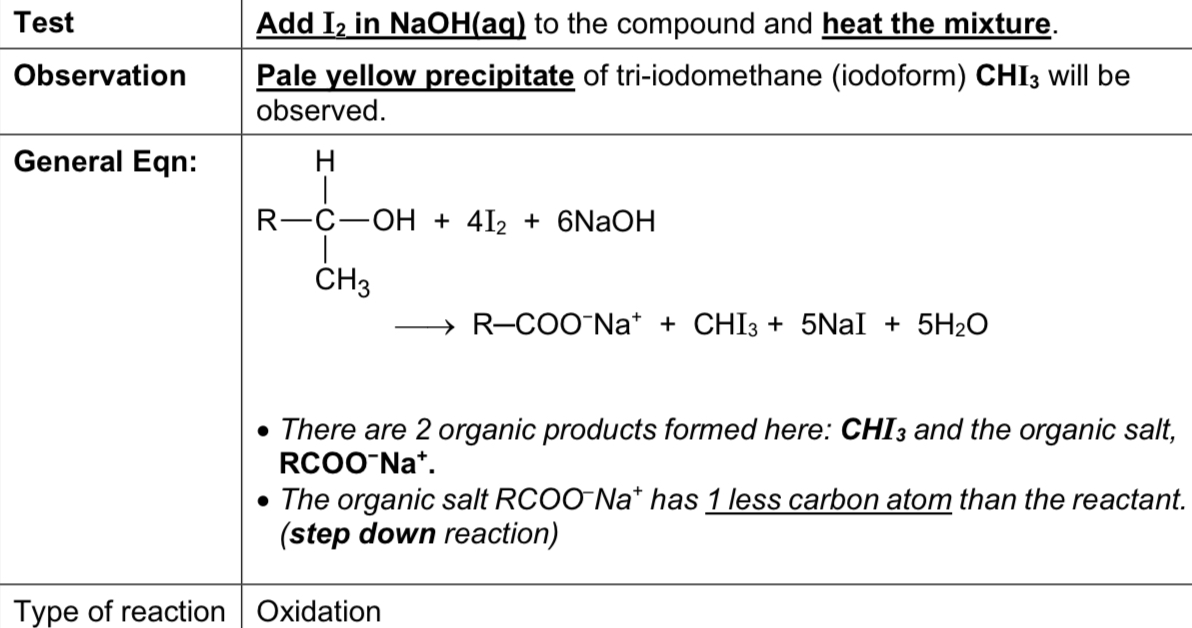
Describe iodoform test
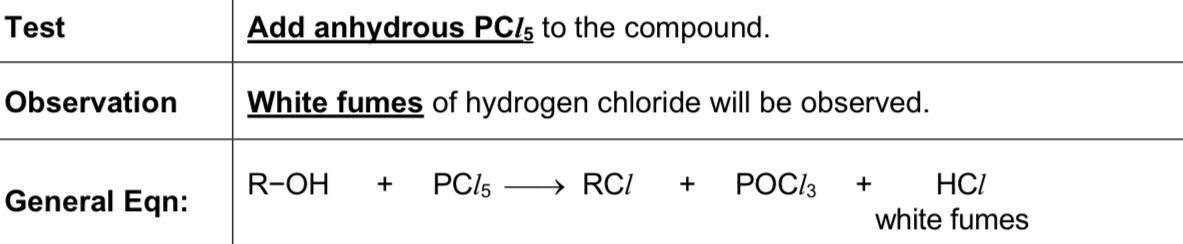
Describe test using PCl5
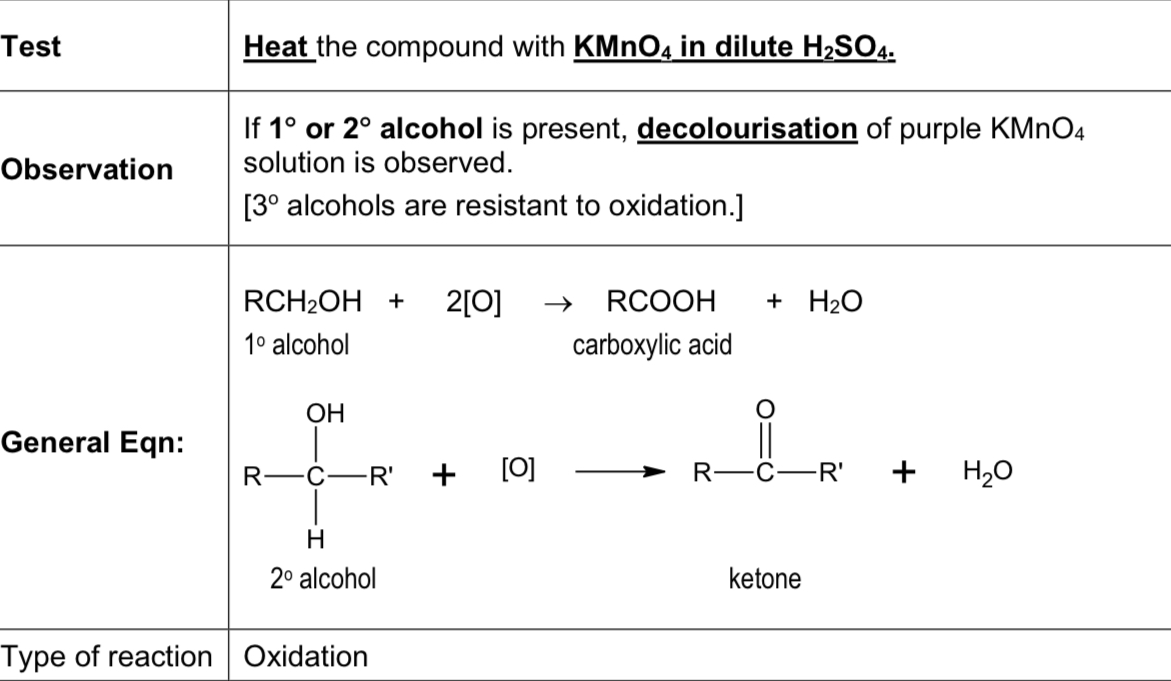
Describe test using KMnO4
What are aryl alcohols?
aromatic compounds containing the −OH group that is not directly bonded to the benzene ring
What are phenols?
–OH group attached directly to the benzene ring contain the phenol functional group
What are the physical properties of phenol?
exists as a crystalline solid at room temperature as a high amount of energy needed to overcome the strong hydrogen bonds between phenol molecules.
caustic and poisonous
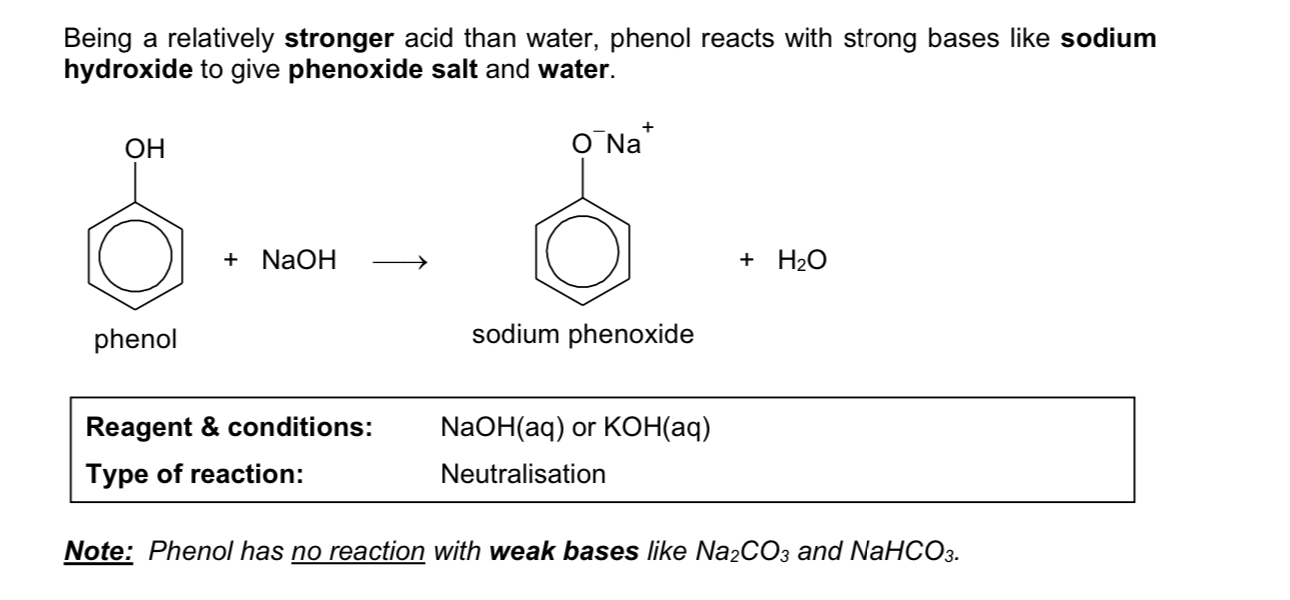
Describe acidity of phenols
relative acidity: Ethanol < Water < Phenol
Explain acidity of phenols
phenoxide ion, the lone pair of electrons on the oxygen atom is delocalised into the benzene ring
this results in the dispersal of the negative charge on the phenoxide ion hence stabilising the phenoxide ion
in comparison, the negative charge in CH3CH2O− is localised on the oxygen atom.
electron-donating alkyl group intensifies the negative charge on the ethoxide ion (CH3CH2O−) hence, destabilising the ethoxide ion
stability of the conjugate base : C6H5O– > OH– > CH3CH2O–
tendency to dissociate and acidity: phenol > water > ethanol
electron withdrawing groups __ acidity
increase
electron donating groups __ acidity
decrease
Explain the effect electron withdrawing groups on acidity
electron withdrawing group, disperses the negative charge on the phenol ion, causing phenol group to lose proton more readily, thus increasing acid strength
Explain the effect electron donating groups on acidity
electron withdrawing group, intensifies the negative charge on the phenol ion, causing phenol group to lose proton less readily, thus decreasing acid strength
acid metal displacement of phenol
neutralisation of phenol
Why is phenol more susceptible to electrophilic substitution compared to benzene?
in phenol, the lone pair of electrons on oxygen atom is delocalised into the benzene ring thereby increasing the electron density in the ring, hence increasing reactivity towards electrophiles
therefore, milder conditions are required for electrophilic substitution reactions of phenol.
State the reaction of phenol and acyl choride
phenol does not react with carboxylic acids to form esters
esterification process involves a nucleophilic attack by alcohol on the electron-deficient carbon of the –COOH group
phenol is a weaker nucleophile than an alcohol since the lone pair of electrons on the oxygen atom is delocalised into the benzene ring
however, phenol can react with acyl chlorides to form esters
in some cases, before reacting with the acyl chloride, phenol is first neutralised with NaOH (aq), forming the phenoxide ion which is a stronger nucloephille than phenol, thus rate of reaction is increased
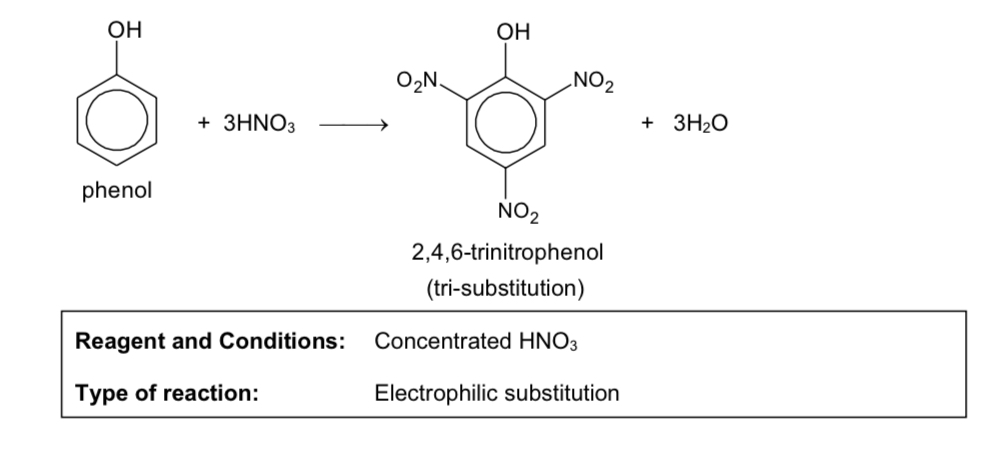
nitration of aromatic ring
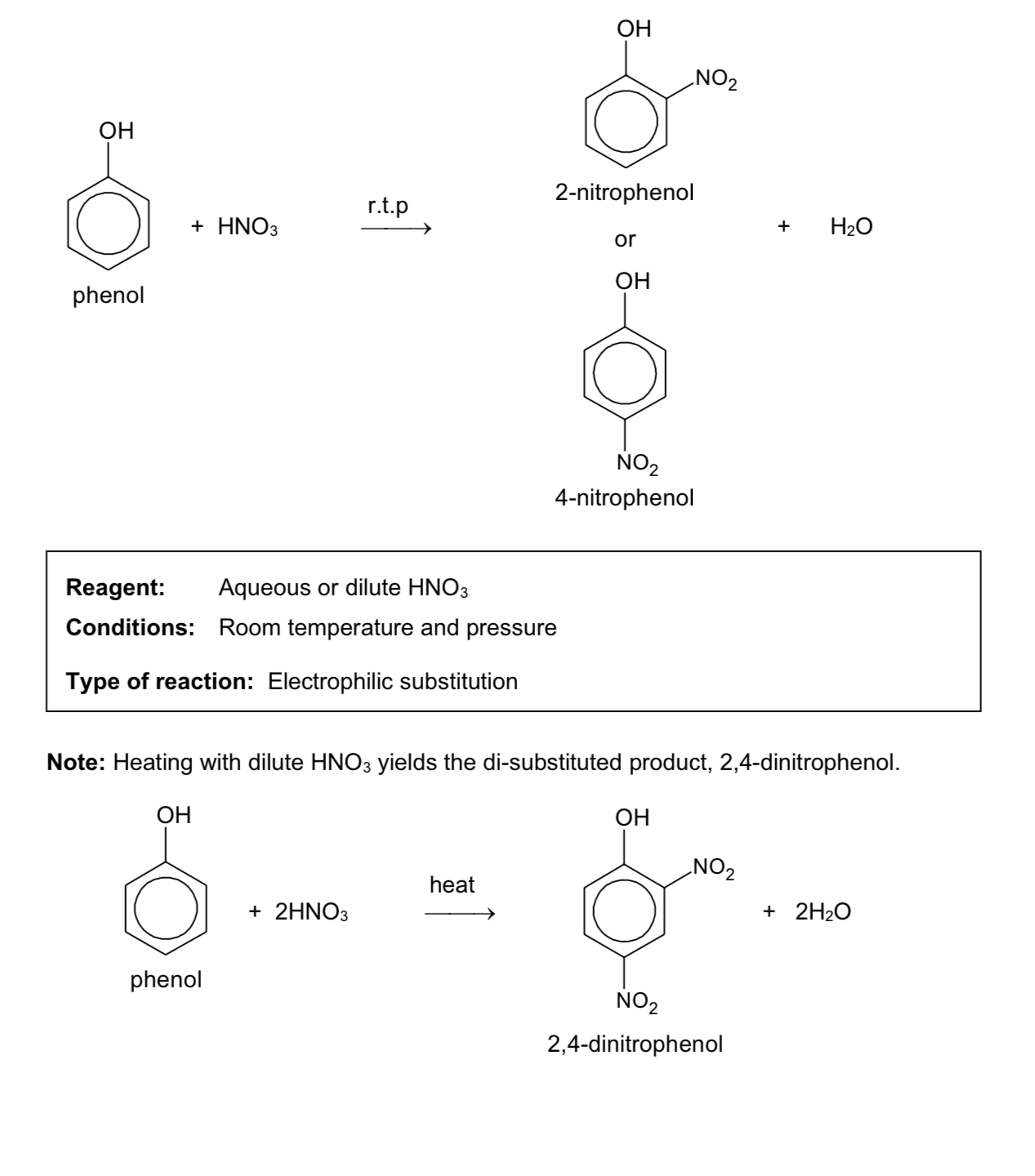
reaction of phenol with X2
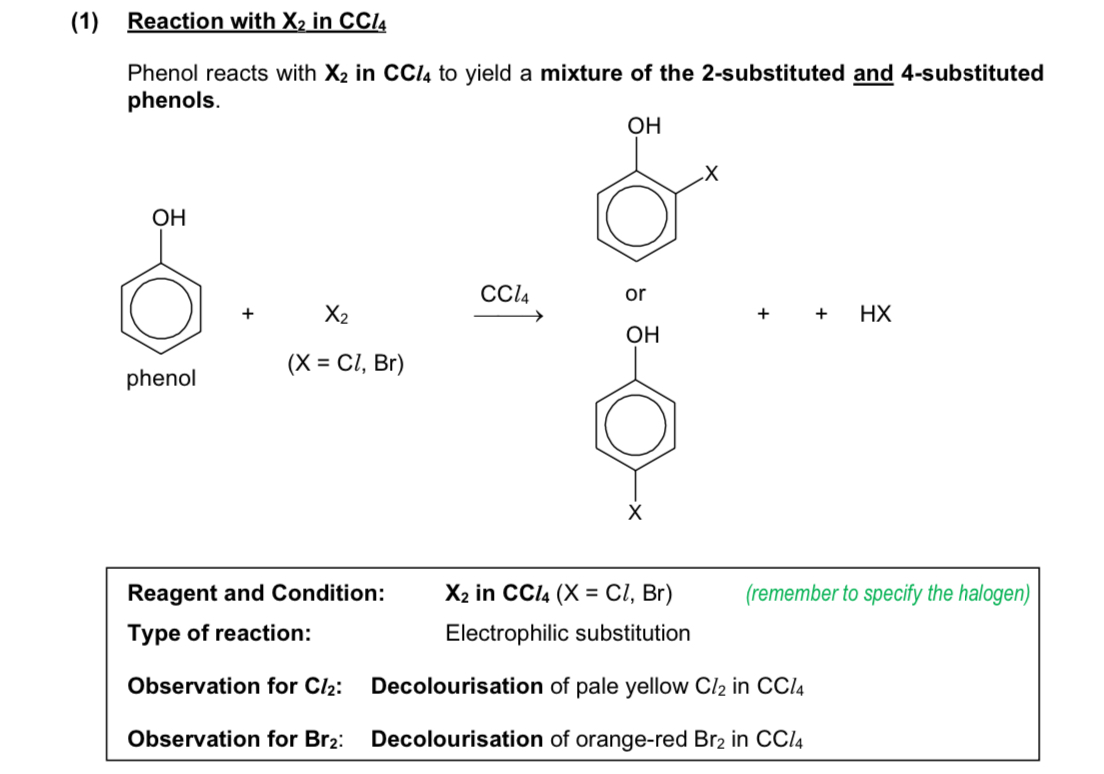
reaction of phenol with X2 in CCl4
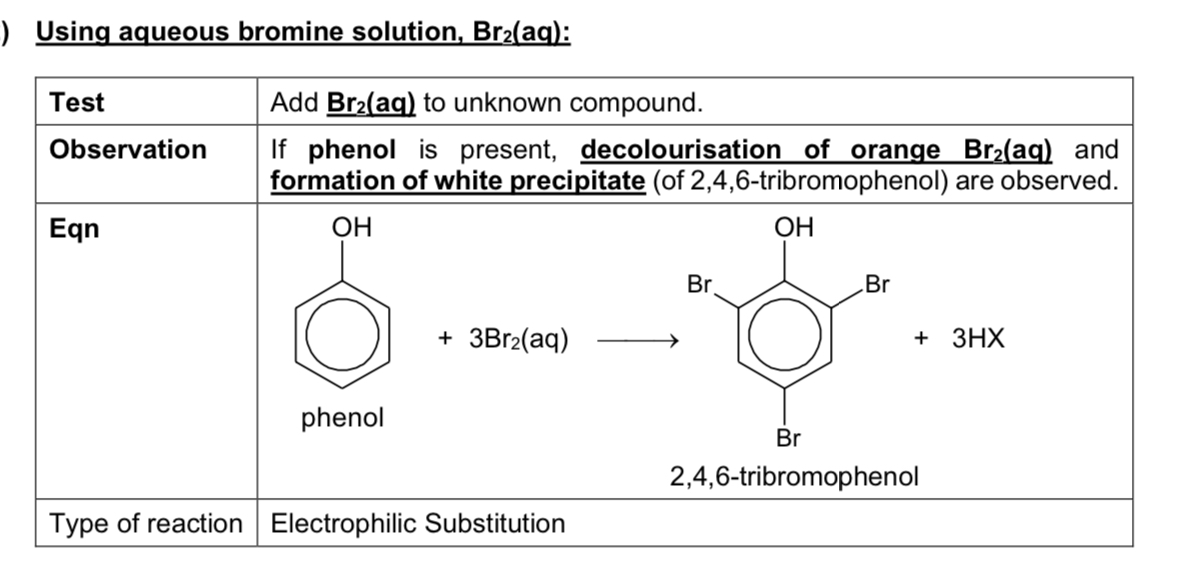
Describe reaction using Br2
Describe reaction using neutral FeCl3

Enthalpy change

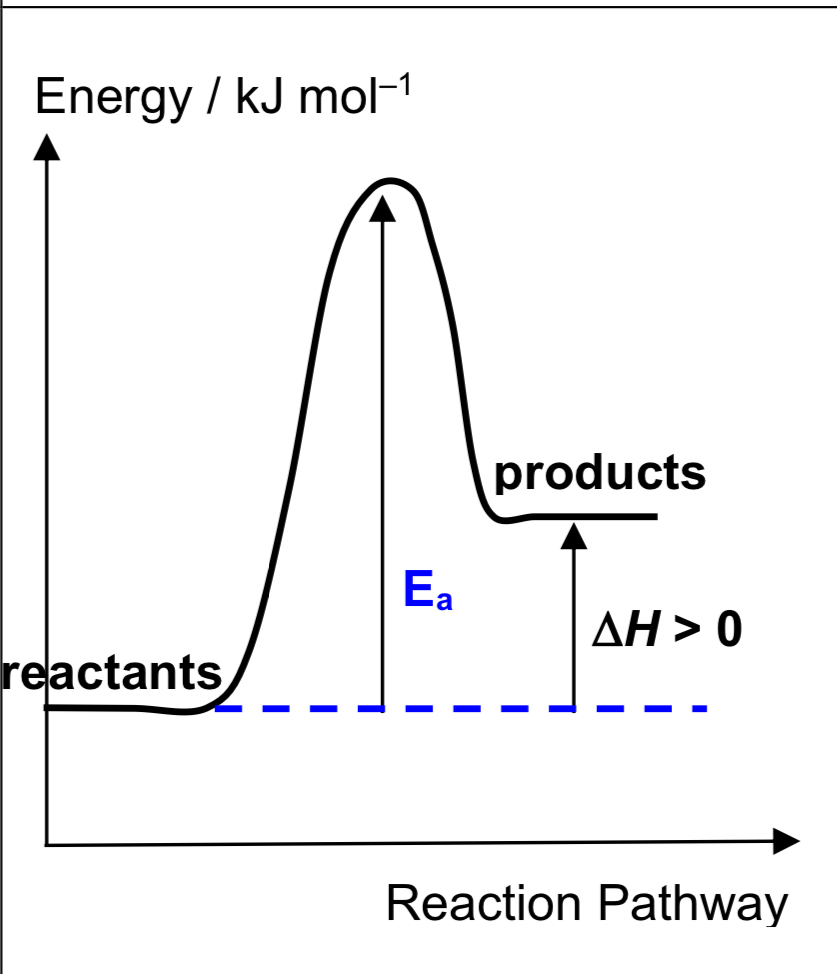
Describe endothermic reaction
temperature of surroundings decreases in the process
Hpdts > Hrxts H > 0 (+ve)
products are thermally less stable than reactants
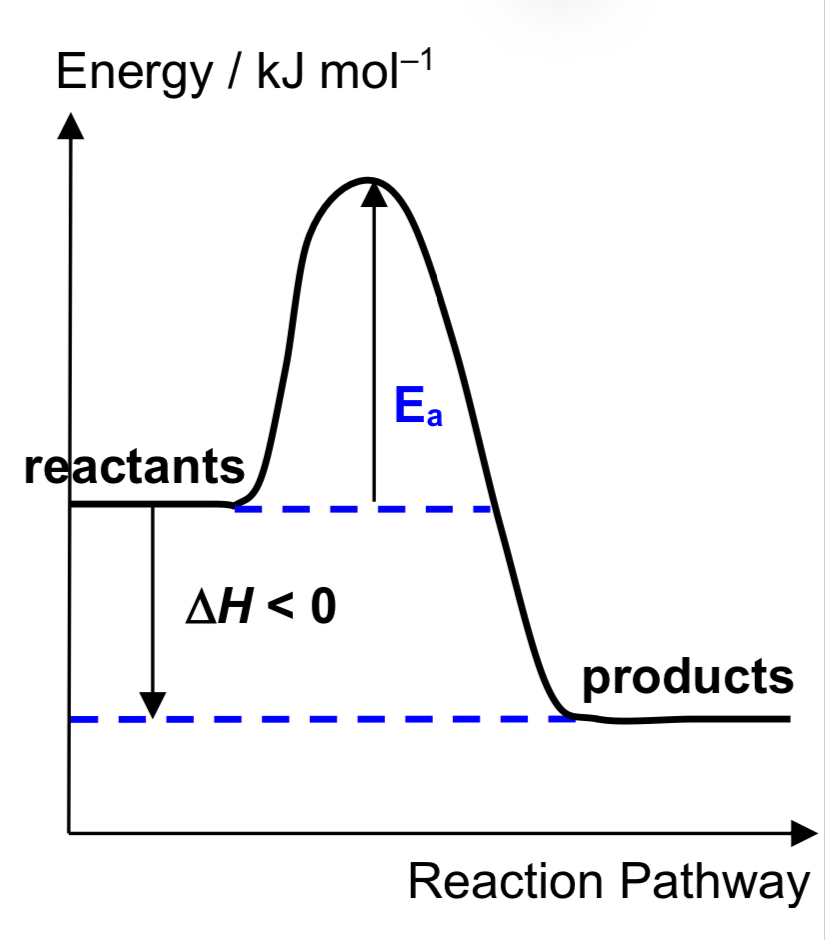
Describe exothermic reaction
temperature of surroundings increases in the process
Hpdts < Hrxts H < 0 (–ve)
products are thermally more stable than reactants.
What is standard enthalpy change
Standard enthalpy change of reaction (H° or H°r) is the amount of energy absorbed or released in a chemical reaction when the molar quantities stated in the chemical equation react under standard conditions of 298 K and 1 bar
What is standard enthalpy change of combustion
Standard enthalpy change of combustion (H°c) of a substance is the energy released when one mole of the substance is completely burnt in oxygen under standard conditions of 298 K and 1 bar
What is standard enthalpy change of neutralisation
Standard enthalpy change of neutralisation (H°n) is the energy released when an acid and a base react to form one mole of water under standard conditions of 298 K and 1 bar.
What is standard enthalpy change of formation
Standard enthalpy change of formation (H°f) of a substance is the energy change when one mole of the substance is formed from its elements under standard conditions of 298 K and 1 bar.
What is standard enthalpy change of atomisation of an element
Standard enthalpy change of atomisation (H°at)!of an element is the energy absorbed when one mole of gaseous atoms is formed from the element in its standard state under standard conditions of 298 K and 1 bar.
What is standard enthalpy change of atomisation of compound
Standard enthalpy change of atomisation (H°at ) of a compound is the energy absorbed when one mole of a compound in a given state is being broken down into its constituent gaseous atoms under standard conditions of 298 K and 1 bar.
What is standard enthalpy change of solution
Standard enthalpy change of solution (H°sol) of a substance is the energy change when one mole of the substance is completely dissolved in a solvent to form an infinitely dilute solution under standard conditions of 298 K and 1 bar.
What is standard enthalpy change of hydration
Standard enthalpy change of hydration (H°hyd) of an ion is the energy released when one mole of the gaseous ions is hydrated under standard conditions of 298 K and 1 bar
What is standard enthalpy change of fusion
Standard enthalpy change of fusion (H°fus) of a substance is the energy absorbed when one mole of the substance is converted from the solid state to the liquid state at its melting point under pressure of 1 bar.
What is standard enthalpy change of vaporisation
Standard enthalpy change of vapourisation (H°vap) of a substance is the energy absorbed when one mole of the substance is converted from the liquid state to the gaseous state at its boiling point under pressure of 1 bar.
What is standard enthalpy change of sublimation
Standard enthalpy change of sublimation (H°sub) of a substance is the energy absorbed when one mole of the substance is converted from the solid state to the gaseous state at a given temperature under pressure of 1 bar.
What is bond energy
Bond energy of a covalent bond is the average energy absorbed to break one mole of the covalent bond in the gas phase into its constituent gaseous atoms under standard conditions of 298 K and 1 bar.