3L - Functional Group Tests
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Baeyer's Test
Test for Unsaturation; detects double or triple bonds in compounds, indicated by a brown precipitate.
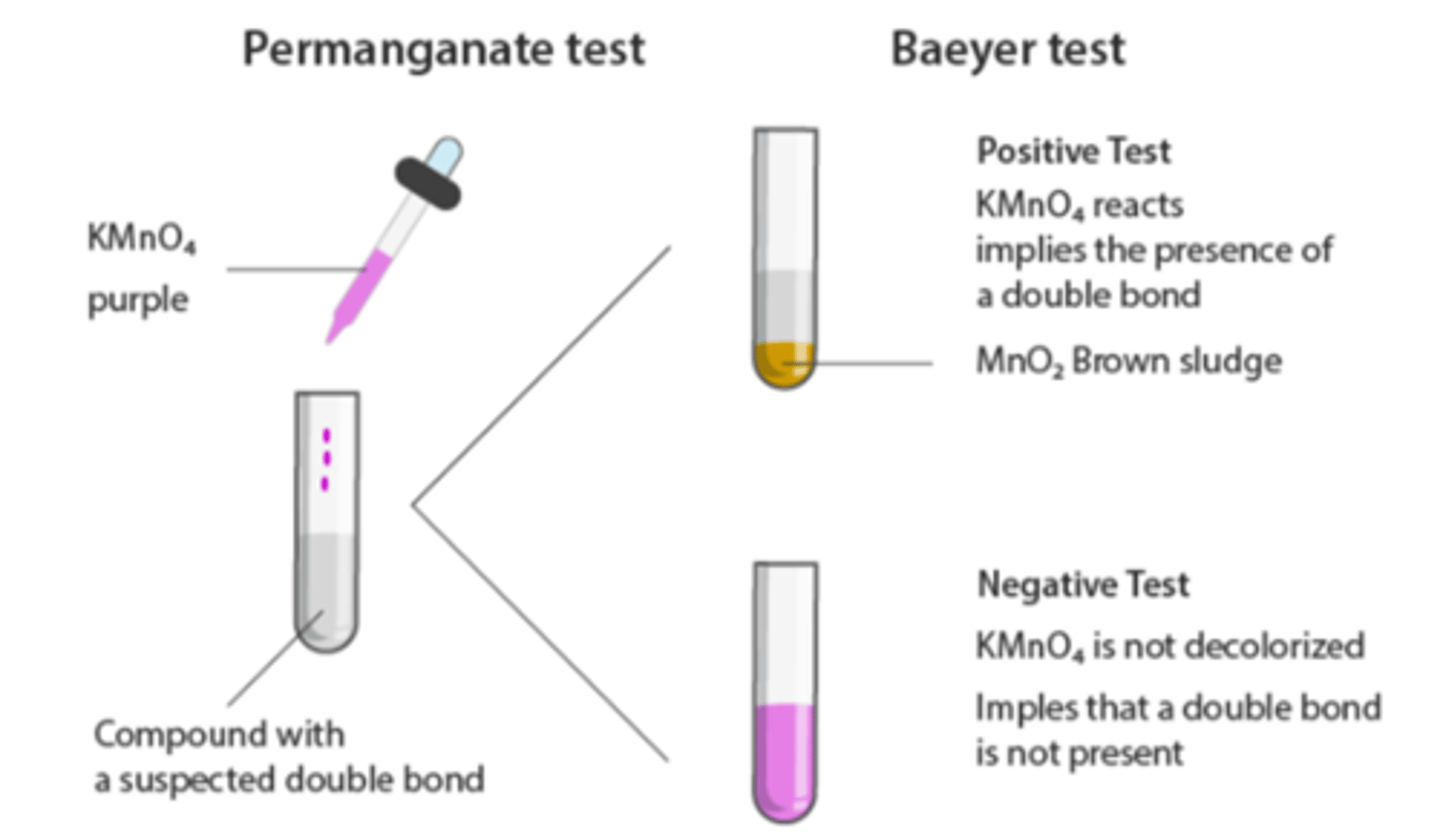
Positive Result in Baeyer's Test
Indicates unsaturation with brown precipitate formed; examples include Turpentine Oil, Olive Oil, Oleic Acid, Castor Oil.
Negative Result in Baeyer's Test
Indicates saturation with no precipitate; examples include Cyclohexane, Butanol, 3-Pentanol, Acetone, Formic Acid, Formaldehyde, Isopropyl Alcohol, Tert-Butyl Alcohol, Acetic Acid, Starch, Heptane.
Iodoform Test
Test for Methyl Ketones (-COCH₃) & Secondary Alcohols; identifies compounds by forming a yellow precipitate.
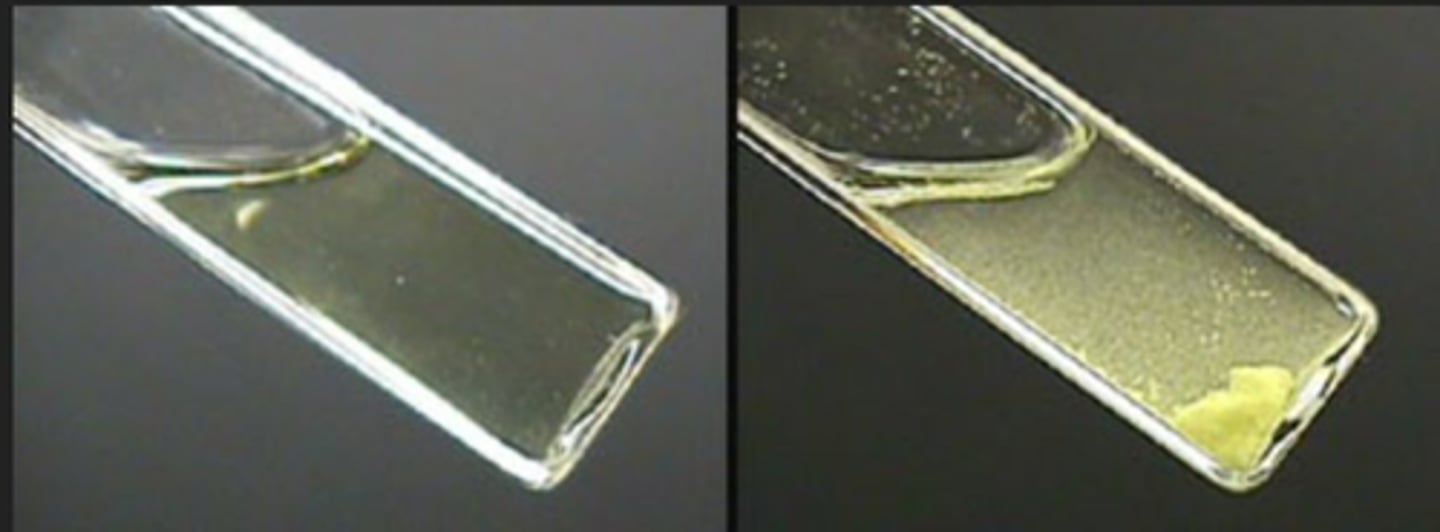
Negative Result in Iodoform Test
Indicates absence of methyl ketones or specific secondary alcohols; all tested samples showed no yellow precipitate.
Fehling's Test
Test for Reducing Sugars & Aldehydes; detects these compounds based on the formation of red or orange precipitate.

Negative Result in Fehling's Test
Indicates no reducing sugar or aldehyde; all tested samples showed no red precipitate.
Lucas Test
Test for Alcohols; differentiates between primary, secondary, and tertiary alcohols by forming cloudiness in Lucas reagent (ZnCl₂ in HCl).

Positive Result in Lucas Test
Indicates alcohol presence with cloudiness; examples include 1-Butanol (Primary Alcohol - Slow Reaction), Isopropyl Alcohol (Secondary Alcohol - Cloudy Reaction), Tert-Butyl Alcohol (Tertiary Alcohol - Immediate Cloudiness).
Negative Result in Lucas Test
Indicates absence of alcohol; examples include Turpentine Oil, Cyclohexane, Olive Oil, Acetone, Oleic Acid, Formic Acid, Formaldehyde, Castor Oil, Acetic Acid, Starch, Heptane.
Sodium Bisulfite Test
Test for Carbonyl Groups (Ketones & Aldehydes); identifies these compounds by forming a white precipitate.
Positive Result in Sodium Bisulfite Test
Indicates presence of ketone or aldehyde with precipitate; examples include Acetone (Ketone) and Formaldehyde (Aldehyde).
Negative Result in Sodium Bisulfite Test
Indicates absence of carbonyl group; examples include Turpentine Oil, Cyclohexane, Olive Oil, Butanol, 3-Pentanol, Oleic Acid, Formic Acid, Isopropyl Alcohol, Castor Oil, Tert-Butyl Alcohol, Acetic Acid, Starch, Heptane.
Schiff's Test
Test for Aldehydes; detects aldehydes by turning purple/magenta when Schiff's reagent is added.

Positive Result in Schiff's Test
Indicates aldehyde presence with magenta or purple color; examples include Turpentine Oil, Butanol, 3-Pentanol, Acetone, Oleic Acid, Formic Acid, Formaldehyde, Isopropyl Alcohol, Castor Oil, Tert-Butyl Alcohol, Acetic Acid, Starch.
Negative Result in Schiff's Test
Indicates absence of aldehyde; examples include Cyclohexane, Olive Oil, Heptane.
Bromine Test
Identifies alkenes and alkynes by observing bromine water decolorization.

Negative Result in Bromine Test
(No Color Change = Saturation) → Cyclohexane, Butanol, Pentanol, Formaldehyde, Tert-Butyl Alcohol, Glacial Acetic Acid, Starch, Heptane
Positive Result in Bromine Test
(Decolorization = Unsaturation) → Turpentine Oil, Olive Oil, Acetone, Oleic Acid, Formic Acid, Isopropyl Alcohol, Castor Oil