Bio- Proteins and Enzymes (B1.1 and C1.1)
1/103
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
104 Terms
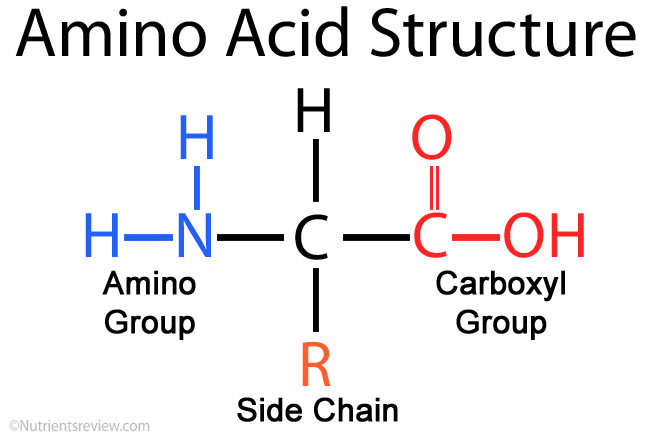
what is the structure of an amino acid?
amine group (NH2) and carboxyl (COOH) on the sides and hydrogen or R group on top/bottom. All attached to a central alpha carbon
what are proteins made up of?
all organisms are made up of the same twenty amino acids (all will have amine group, carboxyl group, and hydrogen attached) (will have diff R groups that will determine the diff chemical properties and behavior)
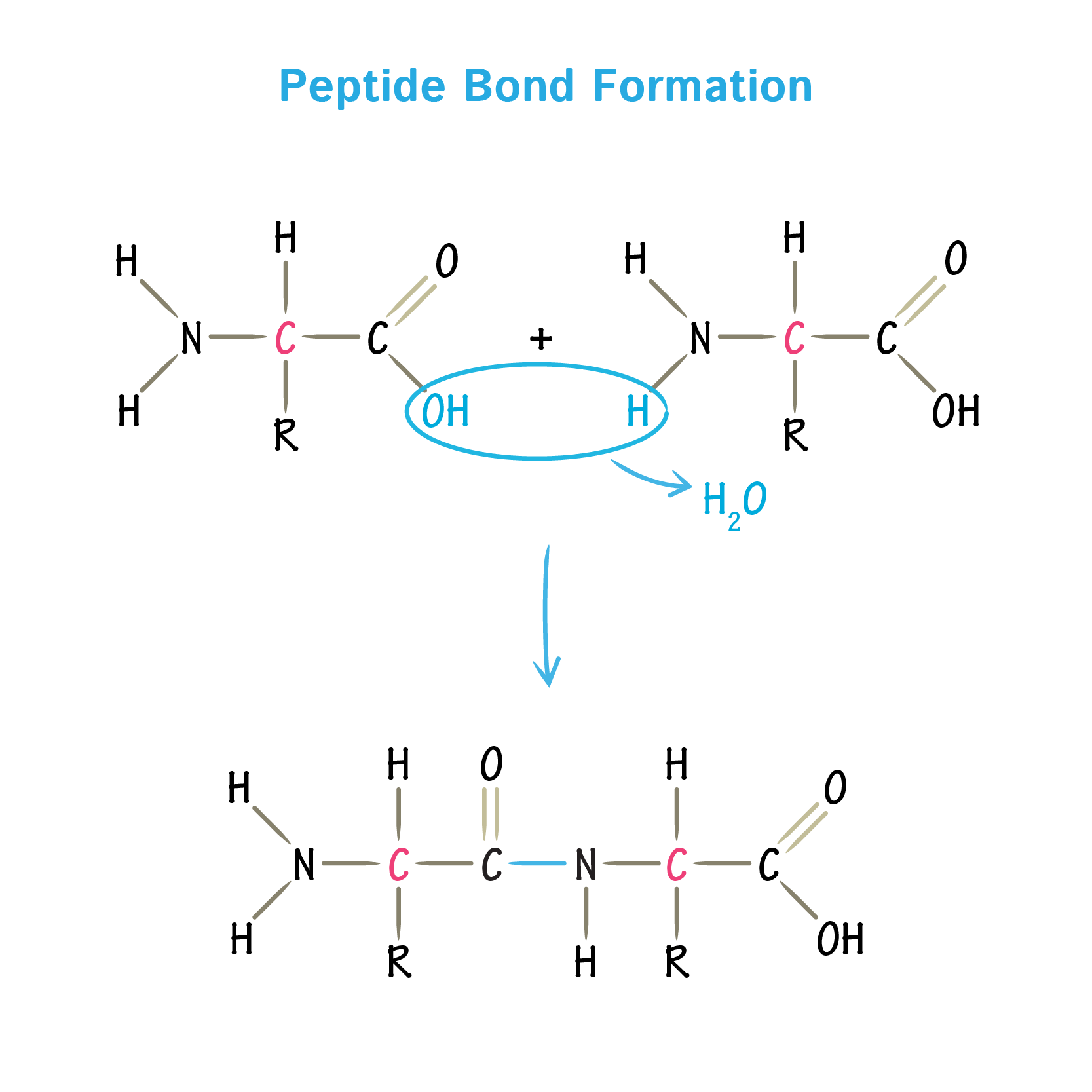
how are dipeptides formed?
amino acid + amino acid → dipeptides + water (condensation reactions)
what is the formation of a dipeptide?
OH and H bond together to form water and the C connects to the N
what is the difference between a protein and a polypeptide?
polypeptide- string of amino acids but is NOT yet functional
protein- string of amino acids but is in functional form
where is the peptide bond always?
between the C and N of neighboring amino acids
where are polypeptides formed?
ribosomes during the process of translation
what are essential amino acids?
amino acids that must be consumed through the diet of a person (9 of the amino acids)
what are non-essential amino acids?
amino acids the body naturally produces (11 of the amino acids can be synthesized)
where are amino acids coded?
in the DNA (genetic code)
how many amino acids can peptide chains have?
any number
true or false: amino acids can be in any order
true
the variety od possible polypeptides is ____
infinite
what is denaturation?
conformational change in the shape of a molecule (like a protein) resulting in the loss of function
when proteins are broken up, what structure is very unlikely to break?
primary structure
what do R groups determine?
chemical properties
what are the two kinds of R groups?
hydrophobic and hydrophilic
R groups can be _____, _____, _____, and/or _____
hydrophobic, hydrophilic, acidic, and/or basic
what determines the functions of proteins?
the shape of the protein
what are gene products?
EVERYTHING
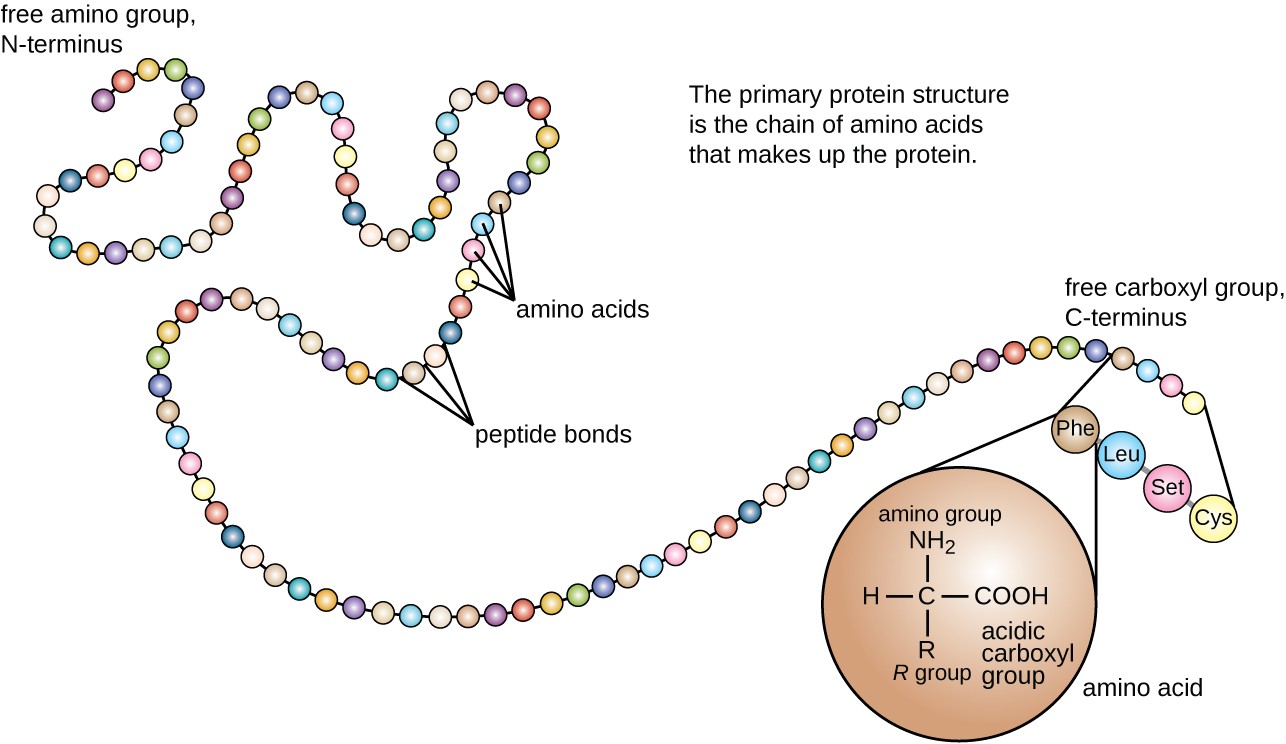
what is the primary structure?
the number and sequence of amino acids in a polypeptide
what determines the three-dimensional shape of proteins?
the sequence of amino acids and the precise position of structure
the precise sequence of amino acids in a polypeptide determines the ____ of the poly peptide due to the reactions between the _____
shape, R groups
what kind of bonds are in the primary structure?
ONLY peptide bonds
what is the protein folding problem?
the challenge of predicting the three-dimensional structure of a protein based solely on its amino acid sequence
the folding process is caused by diff factors and can result in multiple possible structures
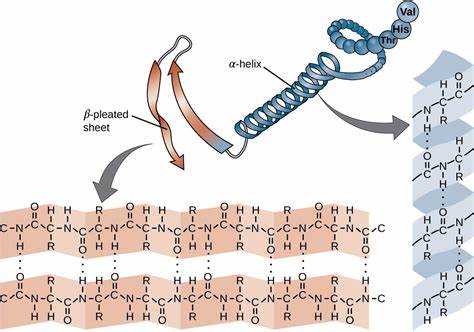
what kind of bonds are in the secondary structure?
ONLY hydrogen bonds
what structures can be formed because of the hydrogen bonds between the C=O of one amino acid and the N-H of a second amino acid
alpha helices
beta-pleated sheets
where are hydrogen bonds formed?
regular intervals
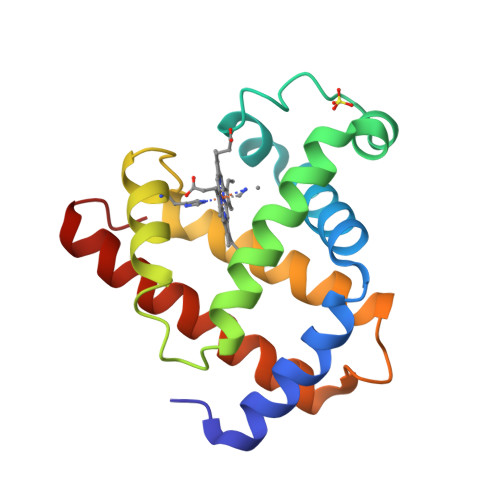
what is the tertiary structure?
further folding of the polypeptide into 3-D structure
because of interactions between the R groups
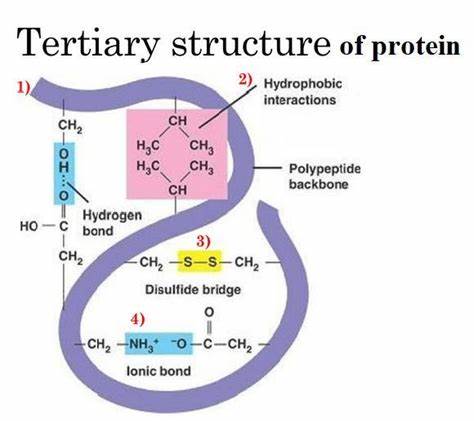
what interactions occur in the tertiary structure?
ionic bonds between charged R groups (amine group becomes positive and carboxyl becomes negative forming ionic bonds)
covalent bonds between R groups (disulfide bonds between 2 cysteines)
hydrogen bonds between polar R groups
hydrophobic and hydrophilic interactions of R groups (proteins in organisms are surrounded by water)
what are disulfide bridges?
form between R groups of two cysteine amino acids in close proximity in a polypeptide
what can happen to carboxyls and amine groups in R groups?
can become positively charged or negatively charged because of binding or dissociation of hydrogen ions causing them to be able to participate in ionic bonding
where are the hydrophobic and hydrophilic amino acids in a protein?
hydrophobic
non-polar R groups
in the core/inside of the globular protein
hydrophilic
polar/ionic R groups
outside the protein
what are integral proteins?
embedded within the phospholipid bilayer
the part of the integral protein that is in the middle of the bilayer is surrounded by hydrophobic fatty acid tails and is hydrophobic because of the non-polar R groups
the part of the integral protein that is exposed to water is hydrophilic because of the polar and charged R groups

what are channel proteins?
have a tunnel lined with hydrophilic amino acids to allow hydrophilic molecules through the bilayer
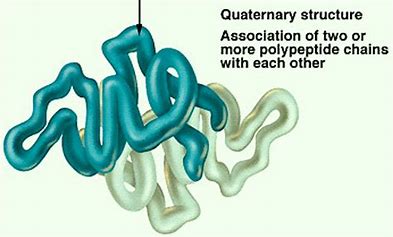
what is the quaternary structure of a protein?
not all proteins have them
proteins composed of more than one polypeptide chain
what is involved with the quaternary structure?
held together due to the R groups on each polypeptide like:
hydrogen bonds
covalent bonds (disulfide bridges)
ionic bonds
hydrophobic/hydrophilic interactions
NOS: what does technology allow?
imaging of structures that would be impossible to observe with the unaided senses
For example, cryogenic electron microscopy has allowed imaging of single-protein molecules and their interactions with other molecules
what is a conjugated protein?
protein attached to a non-polypeptide group known as a prosthetic group (helper molecule)
what is an example of an conjugated protein?
hemoglobin (globular conjugated protein)
two alpha polypeptide chains
two beta polypeptide chains
four heme groups (not polypeptides) (heme= iron groups / make the hemoglobin attract oxygen)
heme group is the helping molecule
function: carries oxygen within red blood cells
what is a non-conjugated protein?
composed of only polypeptides
what are examples of a non-conjugated protein?
insulin (globular non-conjugated protein)
two polypeptide chains linked by two disulfide bridges
function: regulates the blood glucose levels by causing the liver to release glucose from the blood
collagen (fibrous non-conjugated protein) (shown in the pic)
three polypeptide chains that are tightly coiled together into a triple-helix structure
has beta-pleated sheets that form the helical structure
function: main structural protein found in connective tissue like skin, cartilage, and bones
the fibrous nature provides strength and elasticity to tissues
what are the similarities between insulin and collagen?
composed of amino acids joined by peptide bonds during the process of translation on ribosomes
they have a quaternary structure with more than one polypeptide
what are the differences between insulin and collagen?
insulin
globular
spherical
irregular amino acid sequence with hydrophobic amino acids in the core
soluble in water (polar)
2 polypeptides held together by disulfide bridges
functional
hormone with a specific globular shape with a binding site for receptors on target cells
collagen
fibrous
long and narrow
repetitive amino acid sequence
insoluble in water (non-polar)
3 polypeptides held together by hydrogen bonds
structural
3 polypeptides in collagen form flexible fibers with high tensile strength and elasticity which provide structural support to body tissues
(the function is to provide support)
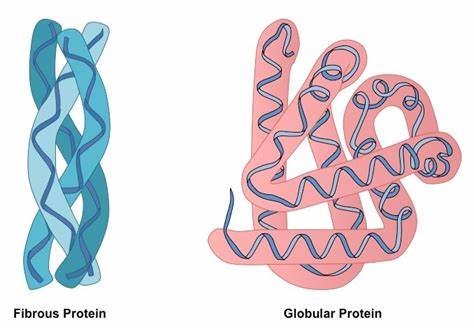
what are the differences between globular and fibrous protein shapes?
Differences
Shape: Globular proteins have a compact, spherical shape, while fibrous proteins have an elongated, thread-like shape.
Solubility: Globular proteins are generally soluble in water, while fibrous proteins are often insoluble.
Function: Globular proteins are involved in enzymatic reactions, transport, and regulation, while fibrous proteins provide structural support and stability.
Structure: Globular proteins have a complex and irregular three-dimensional structure, while fibrous proteins have a repetitive and extended structure.
primary = 1
secondary = 2
tertiary = 3
quaternary = 4
peptide bonds
hydrogen bonds
ionic / disulfide bridges / hydrophobic + hydrophilic reactions
more than one strand
DNA codes proteins so….
enzymes are also from DNA because they are proteins
what are enzymes?
globular proteins
catalysts in metabolic reactions
what are catalysts?
speed up rate of reaction without changing the structure
what is metabolism?
all the chemical reactions that occur in living organisms
what happens without enzymes in metabolism?
metabolic reactions occur slowly, if at all, at body temperature
true or false: each enzyme is specific and catalyzes ONE specific chemical reaction
true
ex. catalase (one enzyme) → hydrogen peroxide (one chemical reaction)
the cell can do what to metabolism through the use of enzymes?
control
true or false: because of enzyme specificity only one enzyme is required by living organisms
false; many diff enzymes are required by living organisms to control all the different kinds of chemical reactions
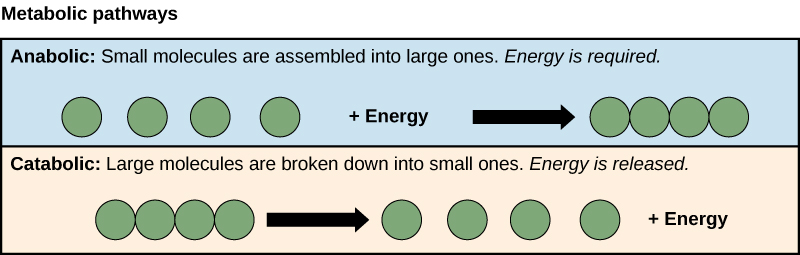
what is anabolism?
storing energy
synthesis of complex molecules from simpler molecules
formation of macromolecules from monomers by condensation reactions
includes:
protein synthesis
glycogen formation
photosynthesis
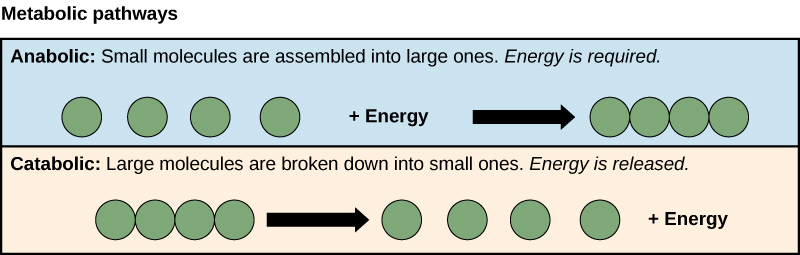
what is catabolism?
releasing energy
breakdown of complex molecules into simper molecules
hydrolysis of macromolecules into monomers
includes:
digestion
oxidation of substrates in respiration
what is the active site?
region where substrates bind and are catalyzed to products
has to have a complementary shape that allows the substrate to bind (like puzzle pieces)
composed of a few amino acids but the overall 3-D shape ensures that the active site can catalyze the reaction of the substrate
what do enzymes act on in a reaction?
substrates
what are substrates?
reactants in enzyme-catalyzed reactions
what do most enzymes end in?
-ase
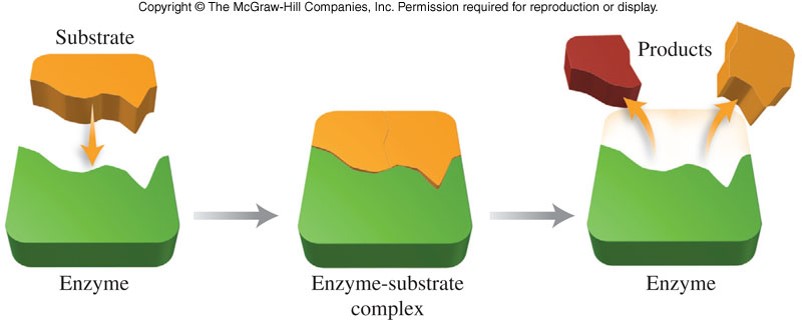
what are the components of the E-S Complex?
(Enzyme-Substrate Complex)
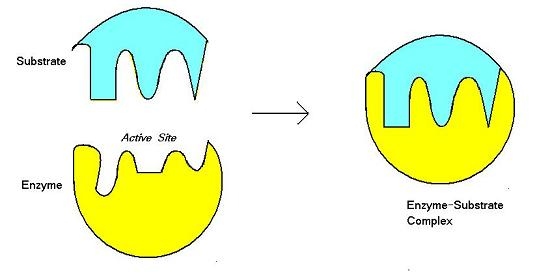
what is the induced fit model?
explains how most substrates are converted to products by enzymes
what happens in the induced fit model?
substrate approaches and enters the active site of the enzyme
the substrate induces the active site of the enzyme to change shape so there is an optimal fit between the substrate and the active site
the product has ___ chemical properties to the substrate and is released from the active site
different
true or false: both enzyme and substrate change shape when binding occurs
true
what is the collision theory?
chemical reactions happen when particles collide
(random movement of particles)
what must particles have in order for reactions to occur?
sufficient energy
correct orientation

particles, including enzymes and substrates, are in what?
constant motion
an enzyme can only catalyze a reaction when the ___ collides with the ___ of the enzyme
substrate, active site
the more frequently substrates collide with active sites of enzymes → the ____ the rate of reaction
faster
what is immobilization of substrates?
substrates are so large they don’t move much and therefore the enzyme must do the moving
what is immobilization of enzymes in membranes?
keeps it in close proximity to the substrate that it catalyzes (the substrate does the moving)
what is enzyme-substrate specificity?
the shape and chemistry of an enzyme’s active site allows one specific type of substrate to be catalyzed
how is an enzyme denatured?
permanent change in the shape of the proteins
results in the loss of the proteins’ biological function
substrates can no longer bind to the active site or catalyze reactions
the active site is the part that gets denatured
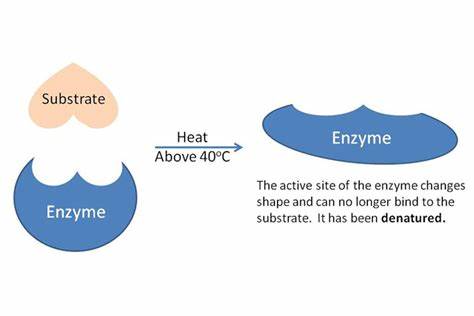
true or false: enzymes are denatured by low temperature
false; they are not denatured by low temperature
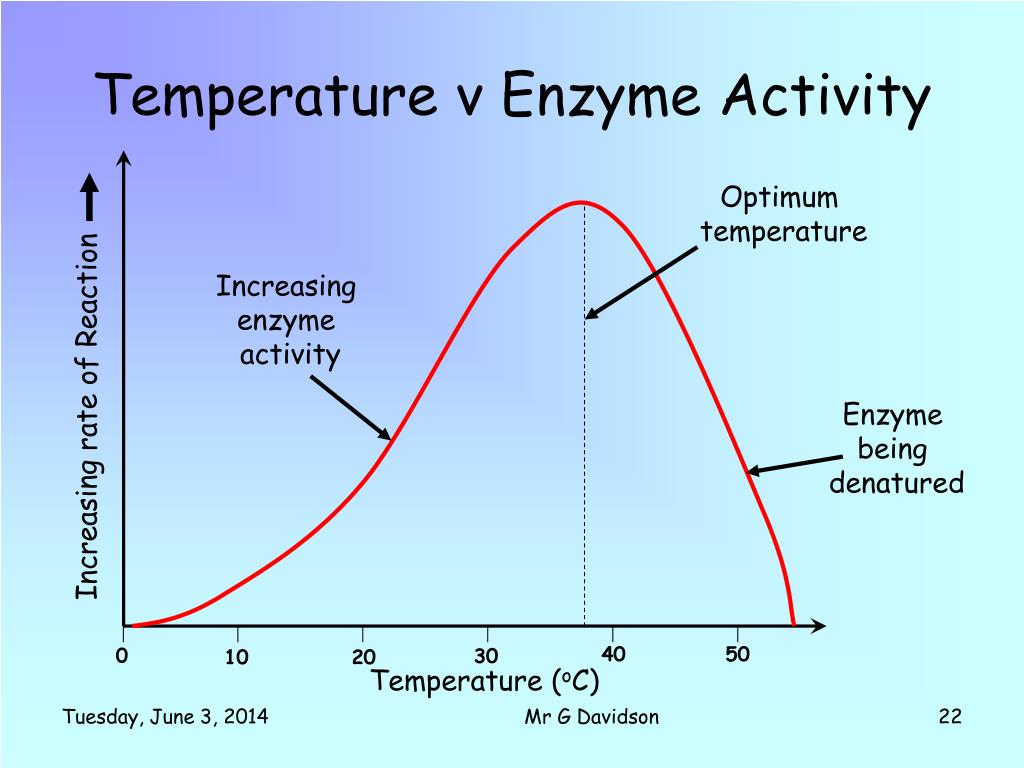
how does temperature affect enzyme activity?
low temperature
kinetic energy of particles is low
number of collisions between substrates and active sites is low
low rate of reaction
(when graph is at 0 → frozen and not denatured)
as temperature increases
particles gain energy and move faster
number of collisions increases
rate of reaction increases until the enzymes’ reach optimum temperature (each enzyme has an optimal temperature)
increase in correct orientations as well
above optimum temperature
bonds in enzyme start breaking
enzyme becomes denatured
substrate is no longer able to bind with active site
rate of reaction drops quickly
each enzyme has a specific optimum temperature
always loss of function because of loss of shape/structure
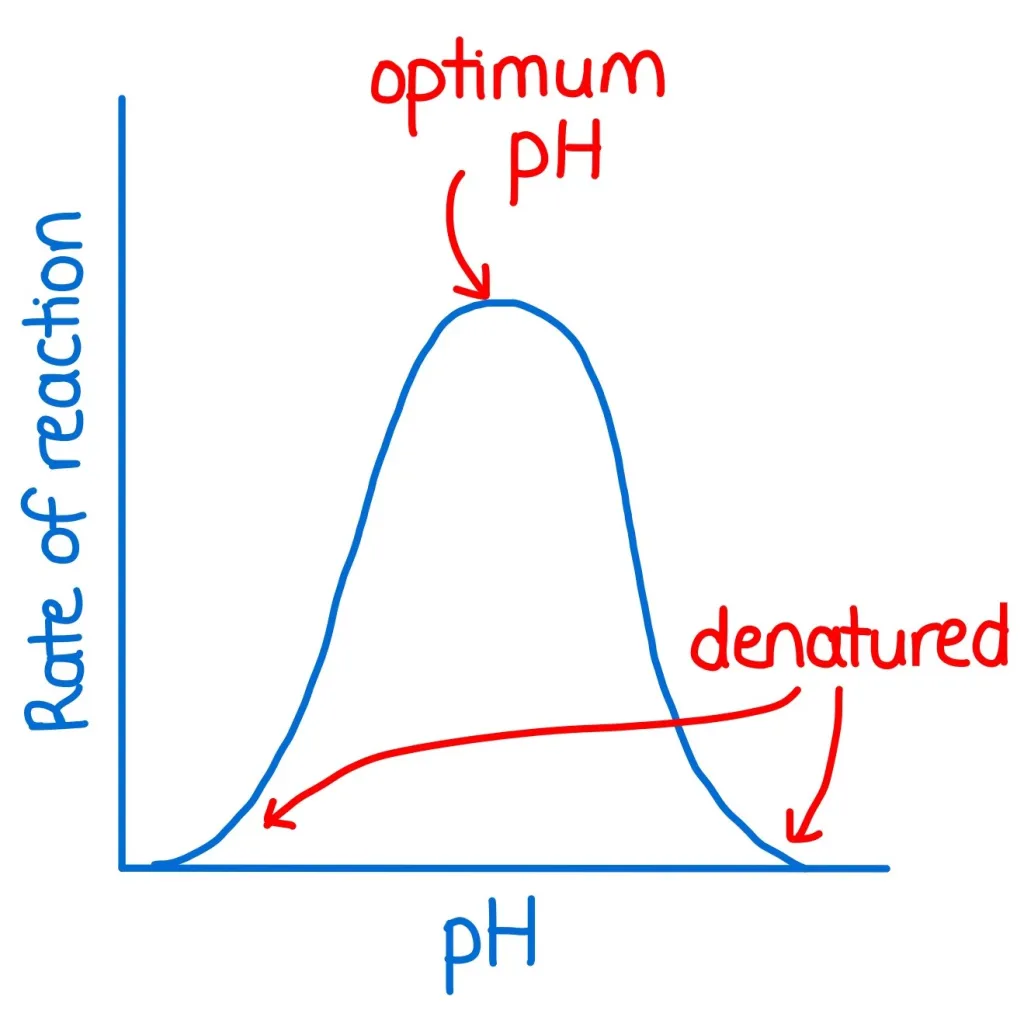
how does pH affect enzyme activity?
all enzymes have an optimal pH
changes in pH affect the shape of enzymes
an increase or decrease in pH away from the optimum results in a decrease in enzyme activity as the active site is no longer as efficient at binding to the substrate
a large change of pH will disrupt ionic bonds and denature the enzyme
the enzyme will stop working as the substrate is no longer able to bind to the active site
diff enzymes have diff optimum pHs
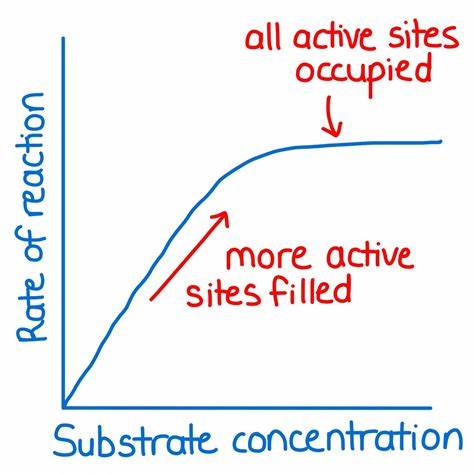
how does substrate concentration affect enzyme activity?
as the substrate concentration increases
increased collisions between substrate and the enzymes’ active sites
rate of reaction increases
reaction rate continues to increase as substrate concentration increases until all of the enzymes’ active sites are saturated (catalyzing as fast as they can because they are surrounded by substrate)
rate plateaus as all enzymes are working at their optimum rate (reaches V-max)
when looking at graphs what do you always assume?
that all other factors and conditions are the same
ex. measuring substrate concentration = same temperature and same pH (and vice versa)
NOS: the generalized sketches of enzyme activity represent scientific models which can be used to develop hypotheses when investigating enzyme activity
(the three graphs of temperature, pH, and substrate concentration)
both temperature and substrate concentration have no denaturation while pH is denatured on both ends of the graph
how do you calculate the rate of reaction in enzyme-catalyzed reactions?
rate of reaction = change in reactant or product / time
changes that can be measured include:
mass or volume of the reactants or products
pH (if the reaction causes a change in the pH)
temperature change (as reactions involve a gain or loss of heat)
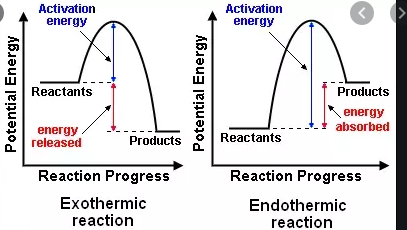
what is activation energy?
the minimum amount of energy required for a chemical reaction to occur
enzymes lower the activation energy for chemical reactions allowing metabolism to occur at body temperature
all chemical reactions require a certain amount of activation energy
more energy is needed without enzymes
less energy is needed for enzyme / catalase reactions
reactants = substrates

what is the transition state of enzymes?
enzymes must reach the transition state before an enzyme reaction goes forward
bonds in substrate are weakened as it binds to active site
highly unstable state between substrate and product
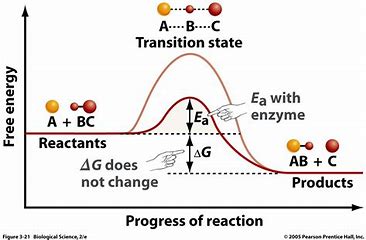
how do enzymes lower activation energy?
energy is required to break bonds in the substrate molecules and the formation of bonds in the product releases energy
enzymes catalyzing a reaction does not affect the change in energy between the substrates and reactants
on graph:
triangleG does not change (free energy)
Ea goes from A + BC to the transition state height wise and is the activation energy
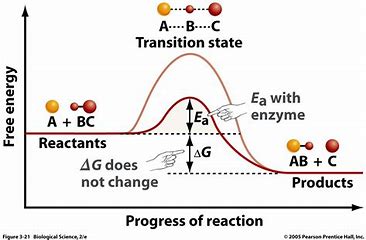
what is exergonic vs endergonic reactions?
exergonic
less energy in products than reactants; ie condensation reactions
exothermic (warmer)
endergonic
more energy in products than reactants; ie hydrolysis
endothermic (colder)
what are intracellular enzymes?
produces on free floating ribosomes (they make proteins) in the cytoplasm
active within the cell
required for most stages of aerobic (cell) respiration
produced by mitochondrial enzymes
required for photosynthesis
produced by chloroplast enzymes
examples of metabolism include:
glycolysis
Krebs cycle of respiration
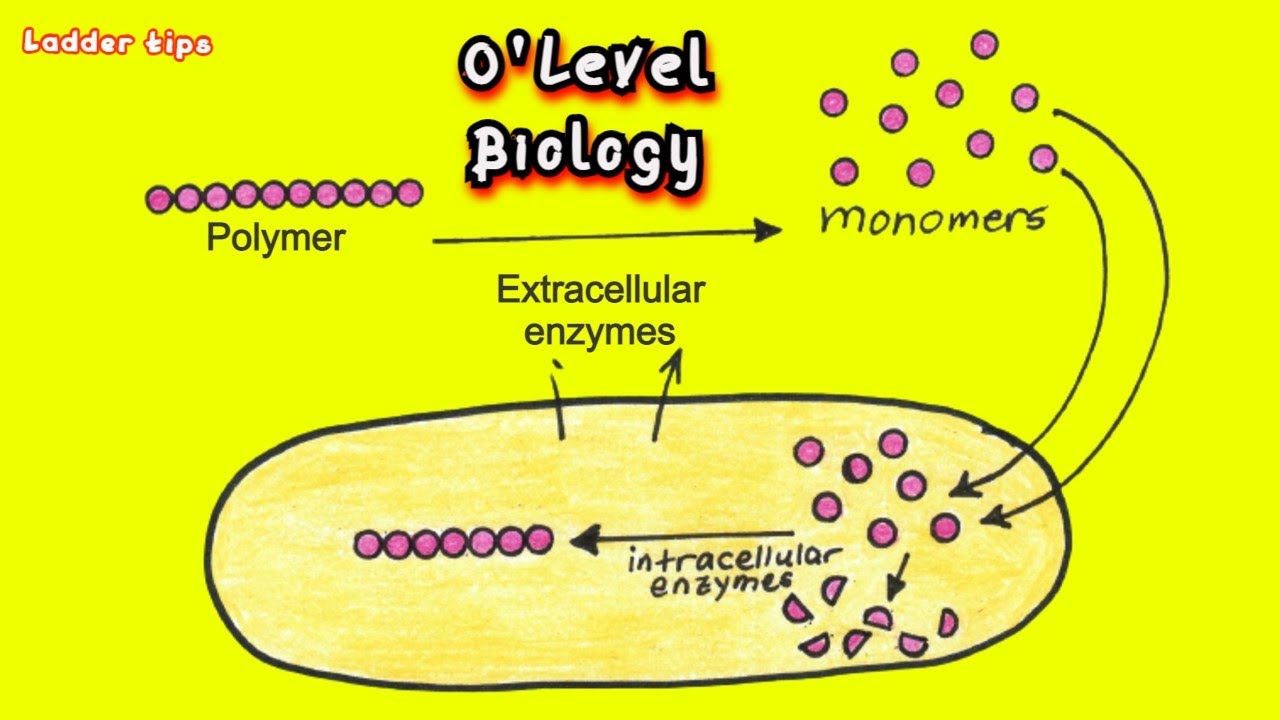
what are extracellular enzymes?
active outside the cell
synthesized by ribosomes attached to the rough ER - proteins sent to Golgi apparatus for processing - packaging in secretory vesicles and released outside of cell in exocytosis
example:
digestion
enzymes secreted from specialized cells into the lumen of the digestive system from the human gut
in photo:
blue near the top = secretory vesicles
green bean shapes = golgi apparatus
spikey caterpillar shapes = rough ER
what is heat generation?
inevitable consequence of metabolism
metabolic reactions are NOT 100% efficient in energy transfer
endotherms depend on the release of heat from metabolism to maintain a constant body temperature
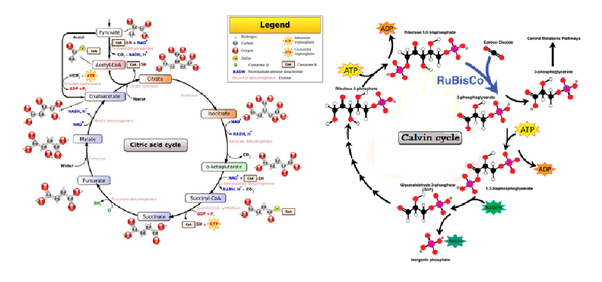
what are the metabolic pathways?
most metabolic reactions happen in a sequence of small steps
two main types of pathways:
linear / chains
cyclical
what is a linear pathway?
glycolysis (breaking up glucose into 2 pyruvate) in respiration is an example of a linear metabolic pathway
all chemical reactions in the pathway require specific enzymes

what is a cyclical pathway?
the krebs cycle in respiration is an example of a cyclical metabolic pathway
the calvin cycle in photosynthesis is an example of a cyclical metabolic pathway
all chemical reactions in the pathway require specific enzymes
(citric acid cycle = krebs cycle)
what is enzyme inhibition?
molecules that stop or slow down an enzyme’s ability to catalyze a reaction
messes with the active site and interferes with the substrates access to the active site
can be:
competitive inhibitors (bind to the active site) (directly)
non-competitive inhibitors (bind to allosteric / alternate site on the enzyme) (indirectly)
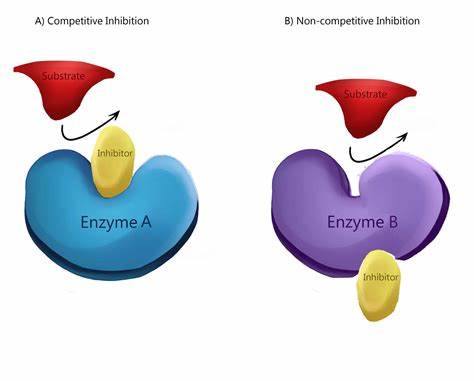
what is competitive inhibition?
have a similar shape and chemistry that mimics the substrate
competes with the substrate and prevents it from binding to the active site
reversible
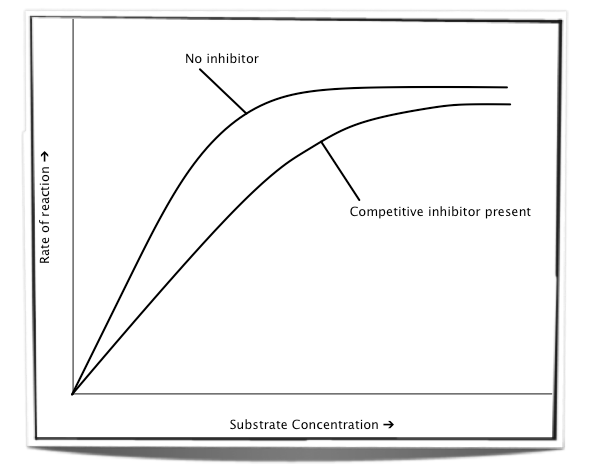
how do competitive inhibitors affect reaction rate?
reduce the reaction rate of the enzyme activity as many of the enzymes have an inhibitor in the active site instead of the substrate
reaction rate increases but at a slower rate
only the substrate concentration changes
it is possible to reach the same rate as with no inhibitor
increasing substrate concentration increases the rate of enzyme activity
enzymes have a higher chance of colliding with the substrate than the inhibitor
the graph eventually plateaus
what are examples of competitive inhibitors?
statins
used to treat high cholesterol levels
if no statins are present:
enzyme HMG-CoA reductase converts HMG-CoA into mevalonic acid
mevalonic acid is then converted into cholesterol through a series of enzyme-catalyzed reactions
statins are the competitive inhibitors of the enzyme HMG-CoA reductase
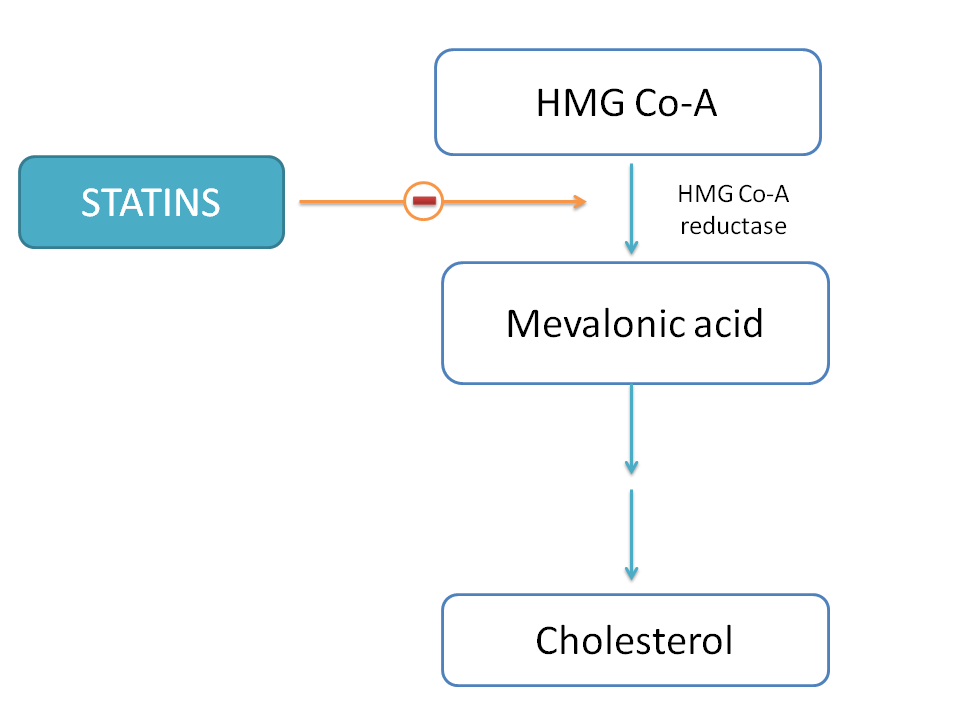
how do statins affect cholesterol?
have a similar chemistry and shape to HMG CoA
binds to the active site of the HMG CoA reductase enzyme
competitively inhibits the production of mevalonic acid
if there is less mevalonic acid produced - there will also be less cholesterol produced
what are non-competitive inhibitors?
different in structure to the substrate (doesn’t mimic)
binds to allosteric site on the enzyme
changes the shape of the active site and prevents the substrate from binding
reversible
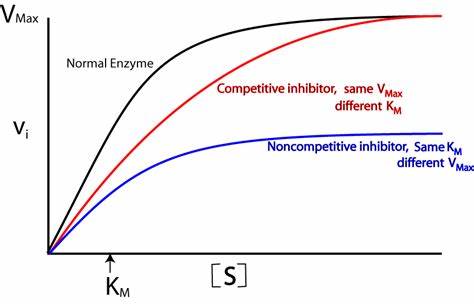
how does non-competitive inhibition affect reaction rate?
reduces the number of functioning enzymes
reduces the reaction rate
increasing substrate concentration only increases reaction rate to a point because of the inhibitors
can never reach V max like competitive inhibition
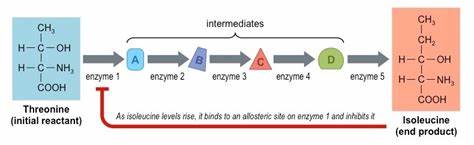
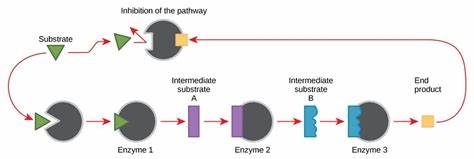
what is feedback/end product inhibition?
final product binds to allosteric site of first enzyme in reaction
active site changes shape, 1st intermediate substrate can’t bind
prevents the rest of reactions in pathway from occurring
pathway starts again when concentration of final product is low and no longer inhibits the first reaction in the pathway
on photo:
green triangle = substrate 1
sub. 1 connects to enzyme 1
purple rectangle = product from ES1
purple rectangle = becomes sub 2 for enzyme 2
and continues until
the yellow square = end product
yellow square = becomes the inhibitor
what is an example of end product inhibition?
isoleucine inhibits threonine deaminase
amino acid threonine is converted to the amino acid isoleucine through a series of enzyme-catalyzed reactions
isoleucine is a non-competitive inhibitor of the enzyme threonine deaminase that catalyzes threonine
if there is an excess of isoleucine it binds to the allosteric site of threonine deaminase causing the active site to change shape and the enzyme is no longer able to catalyze the first reaction in the pathway
if the first reaction in the pathway is not working all reactions in the pathway stop working
when there is not an excess of isoleucine then it is released from the enzyme threonine deaminase and the isoleucine production restarts
threonine is the substrate and isoleucine is the product that becomes the inhibitor
in the photo:
enzyme 1 = threonine deaminase
A = product from ES1 and new substrate for enzyme 2
and so on