Adaptive Immunity 3: B cells
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
95 Terms
Recall the structure of immunoglobin
Nobel Prize (1972) – Edelman & Porter
Which type of cell produces antibodies (immunoglobulins)?
B cells produce antibodies (immunoglobulins).
What is the secreted form of immunoglobulin called?
The secreted form is known as a circulating antibody (Ab).
What is the membrane-bound form of immunoglobulin called?
The membrane-bound form is known as the B cell receptor (BCR).
Do B cells produce antibodies with multiple antigen specificities?
No. Each B cell produces antibodies with a single antigen specificity.
What is special about the range of antibodies produced by B cells overall?
B cells collectively produce a vast array of antibodies with diverse antigen specificities.
Which was the first specific immune recognition structure to be characterised?
The antibody (immunoglobulin) was the first specific immune recognition structure to be characterised.
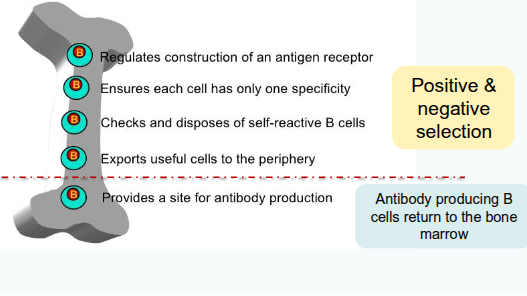
Where do B cells develop?
bone marrow
What signals the development of lymphocyte progenitors from haematopoietic stem cells?
Bone marrow stromal cells signal the development of lymphocyte progenitors and their differentiation into B cells
Which immunoglobulins are expressed on the surface of immature B cells?
Immature B cells express surface immunoglobulin M (IgM) and immunoglobulin D (IgD).
What process ensures immature B cells are not self-reactive before leaving the bone marrow?
Immature B cells undergo screening for autoreactivity through positive and negative selection, known as central tolerance
Where do B cells mature into antibody-producing cells?
B cells mature into antibody-producing cells in the periphery.
What happens to self-reactive B cells that escape the bone marrow?
Self-reactive B cells that leave the bone marrow can still be removed from the repertoire through peripheral tolerance.
B cell development
How is immunological diversity generated in antibodies and T cell receptors (TCRs)?
Diversity is generated by gene rearrangement of gene segments inherited from biological parents.
Does gene rearrangement occur in all genes?
No. Gene rearrangement only occurs for antibodies and T cell receptors (TCRs).
Which gene families contribute to the diversity of TCRs?
The variable (V), diversity (D), and joining (J) gene families contribute to TCR diversity.
How do the V, D, and J gene families generate diversity?
They rearrange to randomly select individual members from each gene family, creating unique combinations.
What causes hypervariability in the TCR’s MHC/peptide-binding region?
Hypervariability arises from differences in the amino acid sequences resulting from gene rearrangement.
How likely is it for the same αβ TCR pairing to be generated in an individual?
It is extremely unlikely for the same αβ pairing to be generated in an individual.
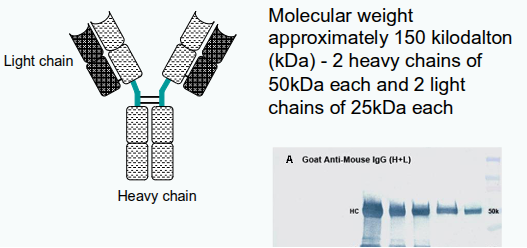
How many immunoglobulin domains are found in each heavy and light chain?
Each heavy chain has 4 domains, and each light chain has 2 domains.
Approximately how many amino acids make up each immunoglobulin domain?
Around 110 amino acids
What are the two types of light chains found in antibodies?
Lambda (λ) and kappa (κ) light chains.
What is the ratio of kappa to lambda light chains in humans?
The κ:λ ratio is approximately 2:1 in humans.
How are the heavy chains in an antibody linked to each other?
Heavy chains are linked to each other by disulphide bonds.
How is each heavy chain connected to its corresponding light chain?
Each heavy chain is linked to a light chain by a disulphide bond.
In a given antibody, are the heavy and light chains identical or different?
In any antibody, the two heavy chains are identical, and the two light chains are identical.
How many identical antigen-binding regions does each antibody have?
Each antibody has two identical antigen-binding regions.
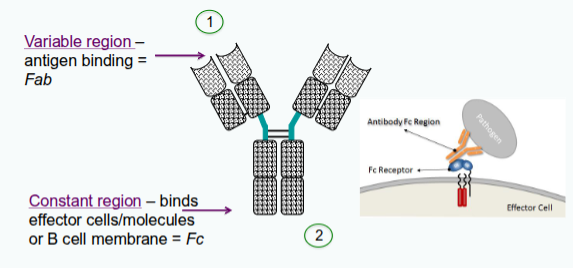
Where is the variable (V) region of an immunoglobulin located?
At the N-terminal end of the heavy and light chains.
What is characteristic of the amino acid sequence in the V region?
It varies dramatically from antibody to antibody.
What do the other domains of the immunoglobulin, apart from the V region, form?
They form the constant (C) region.
What determines the antigen specificity of an antibody?
The unique amino acid sequence in the V regions of its heavy and light chains.
How is sequence variability distributed within the V region?
It is clustered into hypervariable regions (HV1, HV2, HV3).
Which hypervariable region shows the greatest variability?
HV3 is the most variable region.
What are the regions between hypervariable regions called, and how do they differ?
They are called framework regions and show less sequence variability.
What happens when the VH and VL domains of an antibody are paired?
Their hypervariable sites come together at the tip of each arm to form the antigen-binding site.
How many hypervariable regions are present in each VH and VL domain?
Three hypervariable regions are present in each VH and VL domain.
What do the three hypervariable regions from VH and VL together form?
They form a surface that is complementary to the antigen.
What is another name for the hypervariable regions in antibodies?
They are also called complementarity-determining regions (CDRs).
Which hypervariable regions correspond to which CDRs?
CDR1 = HV1, CDR2 = HV2, and CDR3 = HV3.
Where is the antigen-binding site located on the antibody?
At the tip of each arm of the antibody molecule, formed by the paired VH and VL domains.
What forms the antigen-binding site of an antibody?
The hypervariable (HV) regions, also known as complementarity-determining regions (CDRs), form the antigen-binding site.
Do the amino acid sequences of HV/CDRs remain the same in all antibodies?
No. The amino acid sequences of the HV/CDRs in the heavy and light chains differ between antibodies.
What part of an antigen does an antibody recognise?
An antibody recognises only a small region of a large molecule, called the antigenic determinant or epitope
What is another term for an antigenic determinant?
It is also called an epitope.
What is a conformational (discontinuous) epitope?
A conformational epitope is formed by parts of a protein that come together when the protein is folded.
What is a linear (continuous) epitope?
A linear epitope is composed of a single, continuous segment of the target protein.
How do conformational and linear epitopes differ?
Conformational epitopes depend on protein folding to bring distant amino acids together, whereas linear epitopes are formed from a continuous amino acid sequence.
What are the 2 main roles of antibodies?
Two main roles for antibodies:
1. bind specifically to the pathogen/products that elicited response
2. recruit other cells and molecules to destroy the pathogen
Each function – recognition and effector activity - are structurally separated in the molecule
Fc receptors
What activates the T helper cell before it helps B cells?
The T helper cell is activated by dendritic cells.
Where does the activated T helper cell migrate to after activation?
It migrates to the margins of B cell follicles to interact with B cells.
What happens to the B cell after interacting with the T helper cell?
The B cell moves deeper into the follicle, forming the germinal centre.
Which cells does the B cell interact with in the germinal centre?
The B cell interacts with T follicular helper cells and follicular dendritic cells.
What is the purpose of these interactions in the germinal centre?
They optimise B cell memory formation and antibody production.
How do naïve B cells become activated?
Naïve B cells test their B cell receptor (BCR) on whole antigens or intact proteins and become activated.
What happens to B cells after activation?
They leave their quiescent state, initiate cell signalling, and undergo metabolic reprogramming involving extensive transcriptional, epigenetic, and translational changes.
What do these molecular changes in activated B cells affect?
They alter cell surface protein expression and cytokine secretion.
Where do activated B cells and CD4⁺ T cells meet?
They meet at the T–B border.
What occurs at the T–B border between activated B cells and CD4⁺ T cells?
B cells present peptide–MHC (pMHC) complexes to T cells, which provide ‘help’ via ligand–receptor interactions and cytokines to promote B cell differentiation
Where do T and B cells interact during a T-dependent immune response?
At the margins of the T cell and B cell zones in the lymph node.
Which type of T cell helps B cells produce antibodies?
T follicular helper (Tfh) cells.
What do Tfh cells support in B cells?
Rapid proliferation and diversification of B cells.
What are the outcomes of Tfh cell help for B cells?
Production of different antibody types and increased antibody affinity.
What is Signal 1 in B cell activation within the germinal centre?
Antigen binds and cross-links membrane immunoglobulin; peptides from the same antigen are presented on MHC Class II molecules.
What is Signal 2 in B cell activation?
Interaction between CD40 on the B cell and CD40L on the T cell.
What is Signal 3 in B cell activation?
Cytokines produced by T cells that promote B cell proliferation and differentiation
What happens to B cells during proliferation in the germinal centre?
They undergo cell division and introduce mutations while copying their DNA.
Which parts of the antibody genes are affected by these mutations?
The genes contributing to the variable (V) regions of the heavy and light chains.
What is this mutation process in proliferating B cells called?
Somatic hypermutation.
What is the outcome of some mutations introduced during somatic hypermutation?
Some mutations improve the affinity of the antibody for its antigen.
Why is somatic hypermutation important in adaptive immunity?
It enables the selection of B cells producing high-affinity antibodies, enhancing immune effectiveness
Class/isotype switching
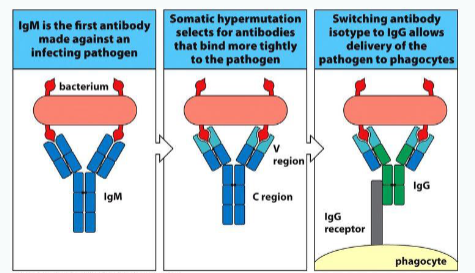
What are the two initial fates of activated B cells?
Some become short-lived antibody-producing cells (plasmablasts), while others enter the germinal centre.
What key process occurs in germinal centres to refine B cell receptors (BCRs)?
Somatic hypermutation, which diversifies the BCR and fine-tunes its specificity
How is antigen specificity maintained after somatic hypermutation?
B cells are re-tested on CD4⁺ T cells to ensure they still recognise the correct antigen.
What are the two main fates of B cells after germinal centre reactions?
Some become memory B cells, while others become long-lived plasma cells that mainly reside in the bone marrow.
What is the function of long-lived plasma cells?
They continuously produce and secrete antibodies, maintaining long-term immunity.
How productive can a fully activated plasma cell be?
A single plasma cell can make and secrete thousands of antibodies per second
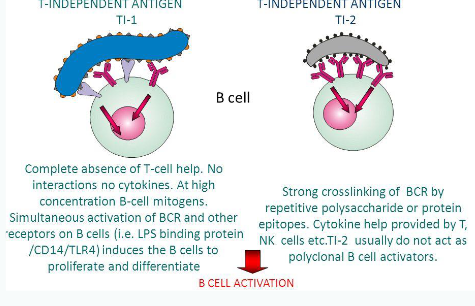
T-independent response
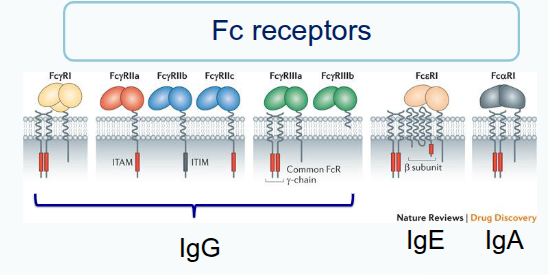
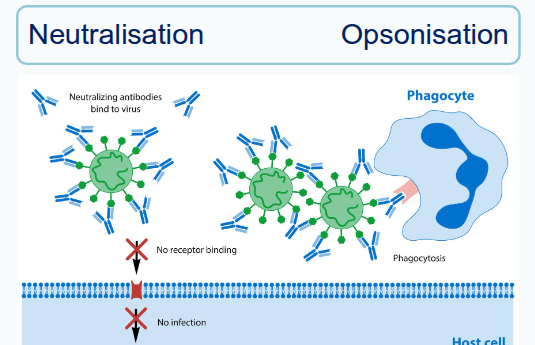
What is opsonisation?
It is the process by which antibodies bind to pathogens, and phagocytic cells with Fc receptors attach to the antibody’s constant region, securing and activating the phagocyte to destroy the pathogen
Which antibody classes can activate the complement system?
IgM and IgG.
What is the result of complement activation by antibodies?
The complement system inactivates and removes antigens or pathogens from circulation.
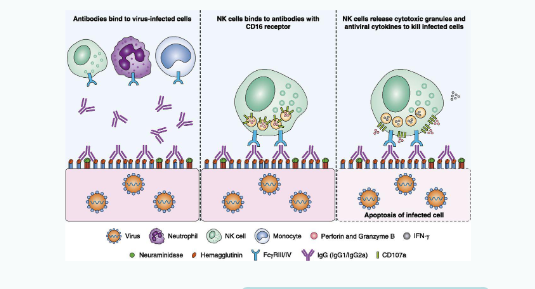
What is antibody-dependent cell-mediated cytotoxicity (ADCC)?
It occurs when antibodies link target cells (e.g. virus-infected cells) to Fc receptors on effector cells such as NK cells, signalling them to attack and kill the target cell.
What is agglutination in the context of antibodies?
It is the cross-linking of epitopes on multiple cells or particles, mediated mainly by IgA and IgM, which limits movement and marks them for destruction.
What is neutralisation by antibodies?
It occurs when antibodies bind to soluble antigens or microbes, preventing them from binding to host cells and thereby blocking their harmful effects.
Which antibody classes are particularly effective in agglutination?
IgA and IgM.
Antibody dependent cellular toxicity (ADCC)
CD16 = Fc receptor for IgG
Antibody dependent cellular toxicity (ADCC)
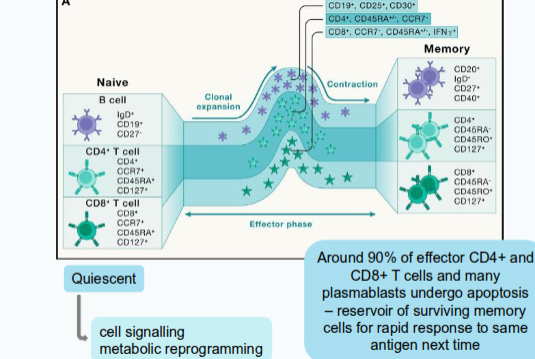
What happens when the immune system encounters a pathogen it has already responded to?
The immune system mounts a faster and stronger response due to immunological memory.
What characterises memory T cells compared with naïve T cells?
They exist at a higher frequency and have intrinsically enhanced function.
What is the role of long-lived plasma cells in immunological memory?
They continuously produce serum antibodies, providing ongoing protection.
How do memory B cells respond to re-exposure to an antigen?
They are rapidly reactivated, proliferate, and generate expanded populations of antibody-secreting cells.
What is the overall benefit of adaptive immune memory?
It ensures a quicker, stronger, and more effective immune response upon re-infection.