Properties of Pure Substances
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
pure substance
a substance that has a fixed chemical composition throughout (homogenous mixture)
is air a pure substance
air is a mixture of several gases, but it is considered to be a pure substance
phase equilibrium
two phases of a pure substrate can exist in equilibrium with a distinct boundary between them
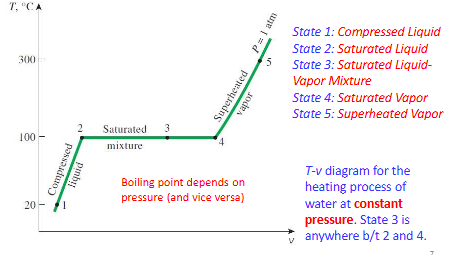
compressed liquid
a substance that is not about to vaporize
saturated liquid
a liquid at Tsat and Psat that is about to vaporize
saturated vapor
a vapor at Tsat and Psat and is about to condense
saturated liquid-vapor mixture
the state at which the liquid and vapor phases coexist in equilibrium
superheated vapor
a vapor that is not about to condense
temperature during a phase change
constant at the saturation temperature
T-V diagram
saturation temperature
the temperature at which a pure substance changes phase at a given pressure
saturation pressure
the pressure at which a pure substance changes phase at a given temperature
latent heat
the amount of energy absorbed or released during a phase change process
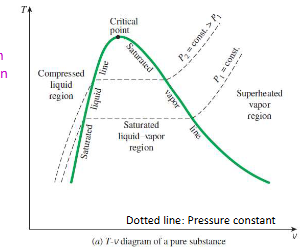
property diagrams
equation of state
any equation that related the pressure, temperature, and specific volume of a substance
substances that cannot use the ideal gas equation
water and R134a
compressibility factor (Z)
a factor that accounts for the deviation of real gases from ideal-gas behavior at a given temp and pressure (PV = ZRT)
when do gases behave as an ideal gas
low densities: low pressure, high temperature