Module 4 - Alkenes
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
What are alkenes and why are they more reactive than alkanes?
Alkenes are unsaturated hydrocarbons that contain one or more C=C bonds.
They are more reactive because of their C=C and normally undergo addition reactions.
What is the general formula of alkenes?
CnH2n
What is the formula of cycloalkenes?
CnH2n-2
What are the bonds present in alkenes?
Sigma bonds ( single covalent bond ) and a pi bond.
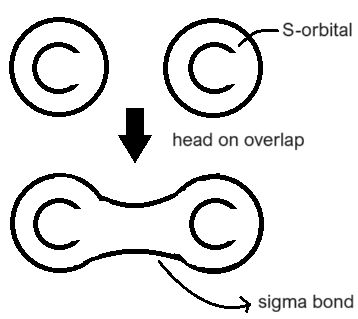
How is a sigma bond formed?
It is formed by the head-on overlap of s-orbitals between two carbon atoms.
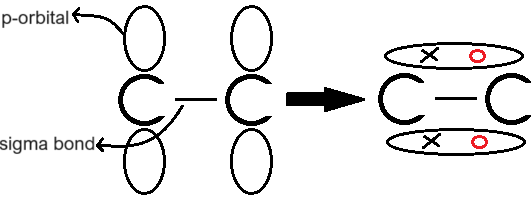
How is a pi bond formed?
It is formed by the sideways overlap of p-orbitals of carbon atoms. This creates a double bond that locks the two carbon atoms in place and prevents the double bond from rotating.
When alkenes react which bond breaks first?
The pi bond breaks first because it has a lower bond enthalpy so the sigma bond remains in place as it is harder to break due to its higher bond enthalpy.
What is the shape of alkenes?
Around their C=C, there are three bonding regions, so the bond angle is 120 and the shape is trigonal planar
Around C-c, there are four bonding regions, so the bond angle is 109.5 and the shape is tetrahedral.
What is sterioisomerism?
Compounds with the same structural formula but different arrangements of atoms in space.
What is E/Z isomerism?
A type of isomerism that only occurs in molecules that contain a C=C bond because this fixes the atoms in one of two possible arrangements.
What must a molecule have to display E/Z isomerism?
A C=C bond that cannot rotate
Each carbon atom must have two different side groups attached
What are the Cahn-Ingold priority rules in regards to E/Z isomerism?
Side groups are given a priority based on what their atomic number is (this is chosen by the first atom attached)
The higher the atomic number, the higher the priority
What molecules display Z isomerism?
Molecules that have their highest priority groups on the same side
→ Zee Same Side
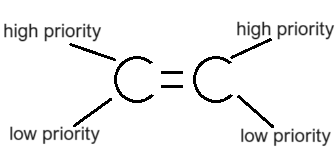
What molecules E isomerism?
Molecules that have their highest priority groups on different sides
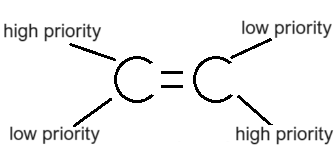
What are the criteria for cis-trans isomerism?
A C=C
Each carbon atoms must be attached to two different side groups
Two of these groups must be the same
What is cis isomerism?
Isomerism where the same groups are on the same side
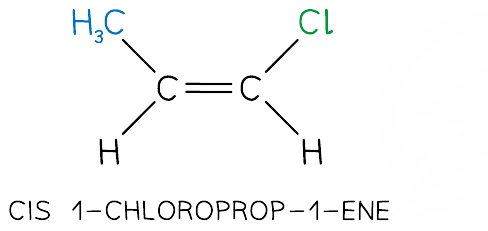
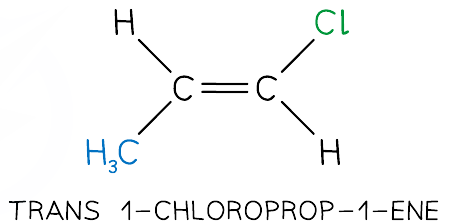
What is trans isomerism?
Isomerism where the same groups are on different sides
What happens during hydrogenation?
Hydrogen adds across the double bond to form a saturated alkane.
Both are in their gaseous states.

What are the conditions of hydrogenation?
Nickle catalyst
Temp of 150
If aqueous, the reaction is happening in water
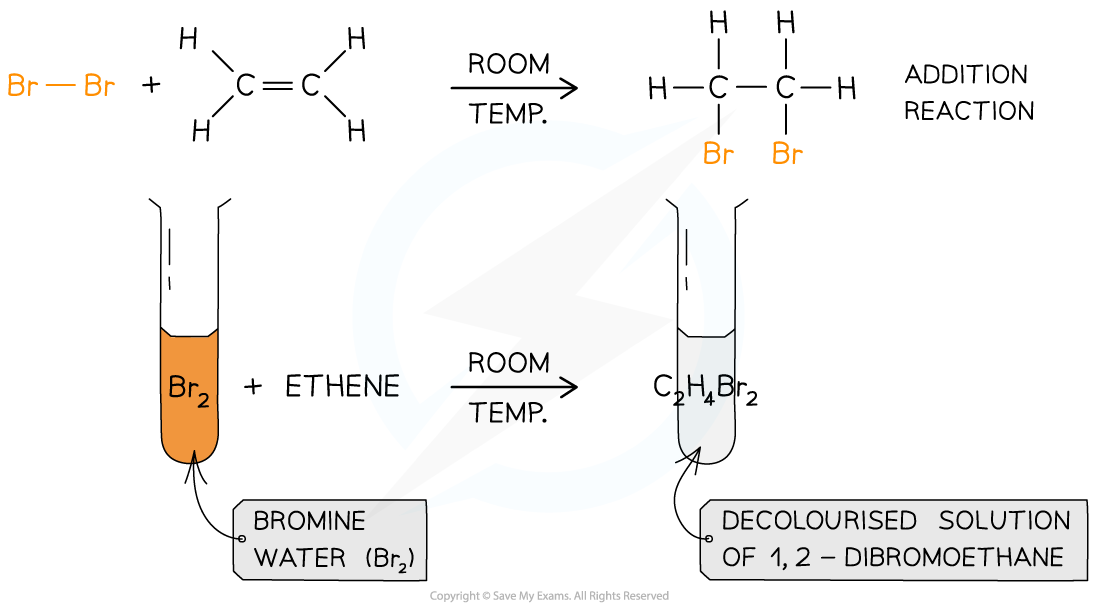
What happens during halogenation?
An alkene reacts rapidly with the halogens at room temperature to form dihaloalkanes

What are the conditions of halogenation?
room temperature
How do we test for alkenes or the presence of a double bond?
Bromine is added dropwise to a same and if the colour changes from orange to colourless (not clear) then it shows a C=C is present.
What happens when alkenes react with hydrogen halides?
Hydrogen Halides (etc. HCl) also react with the alkenes to produce haloalkanes.
The gaseous hydrogen halides are bubbled through liquid alkenes in order to react.

What happens when alkenes react with steam or hydration?
Steam is added across the double bond to form alcohol.
Steam and the gaseous alkene are heated at high temperatures under high pressures in the presence of H3PO4 catalyst.
What are the conditions of the hydration of alkenes?
High temperatures
High pressures
H3PO4 catalyst
What is an electrophile?
An electron pair acceptor
What is a mechanism?
A sequence of steps that show the pathway taken by electrons in chemical reactions.
What does a curly arrow represent?
The movement of a pair of electrons to either make or break a bond
What is heterolytic fission?
The breaking of a covalent bond when one pair of the bonding atoms takes both electrons from the bonded pair.
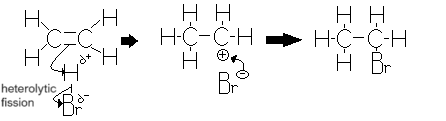
What happens during electrophilic addition reactions?
HBr is a polar molecule as Br is more electronegative than hydrogen causing a dipole to form.
The electron pair in the pi-bond is attracted to the slightly positive hydrogen atom causing the C=C bond to break.
A new bond forms between a carbon atom and the hydrogen atom
H-Br bond breaks by heterolytic fission with the electron pair going to the Br
Bromide ion and carbocation are formed
The +vely charge carbocation attracts the Br- ion to form the product
What is the Markownikoff rule?
If an alkene isn’t symmetrical a mixture of different structural isomers can be formed when a hydrogen halide is added across the double bond.
Structural isomers formed from tertiary carbocations are the major product as they are the most stable and are more likely to be formed
Structural isomers formed from primary carbocations and tertiary carbocations are minor products are they are the least stable.

What are polymers?
They are long chained molecules made from many short chained molecules called monomers in a process called addition polymerisation.
What happens during addition polymerisation?
Many alkenes undergo addition polymerisation to form long saturated chains and it is carried out at high temperatures and pressures using catalysts and occurs from one type of monomer only.
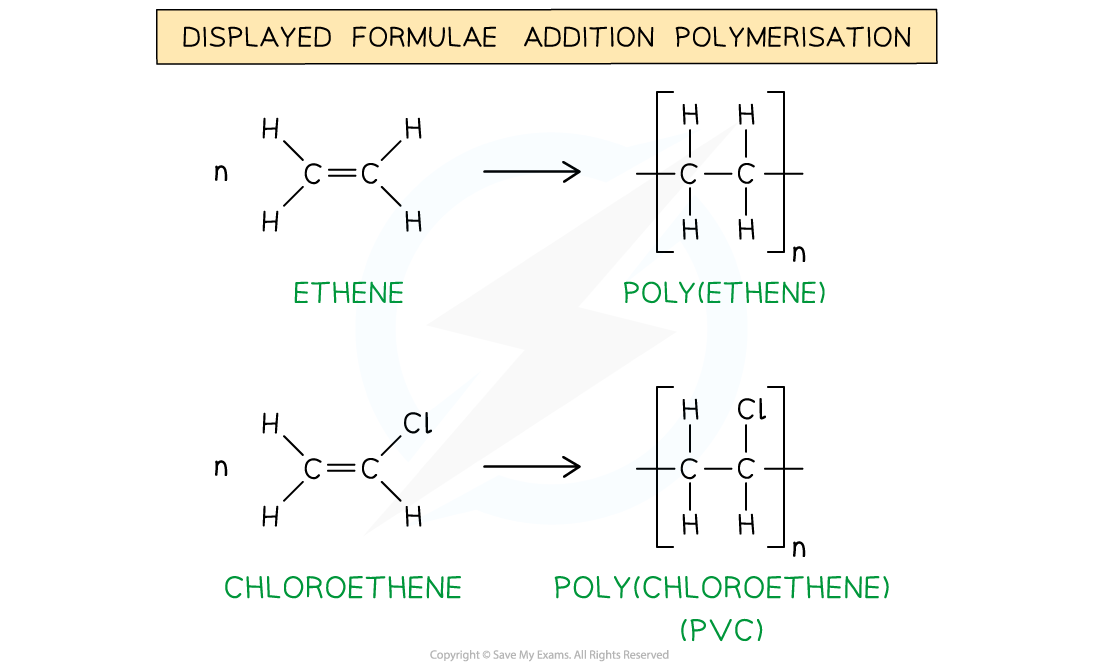
What is a repeat unit?
A specific arrangement of atoms that occur in the structure over and over again and are always placed inside brackets, outside of which is the letter n.
What are the problems with addition polymers?
very stable and don’t break down naturally as they are non-biodegradable
How are addition polymers recycled and sorted?
The best way to protect the environment from plastics is to reuse polymers without processing them.
Collected plastics are sorted into similar types using polymer identification codes
They must be collected together as impurities in the reprocessed polymers will make them useless
How are polymers used for energy production?
Polymers can be burnt to produce heat energy which can be harnessed to make electricity. Agricultural plastics are disposed of this way.
It needs to be a carefully controlled process and polymers containing Cl can produce toxic gas such as HCl so it has to be removed, normally by neutralising it with a base,
How are polymers used as organic feedstock?
Polymers are broken down into new monomers or synthetic gas which can be converted into more useful materials or fuel for oil refineries.
What are biodegradable polymers and what are the benefits?
Polymers are made from natural resources such as starch, maize, cellulose and lactic acid as they will break down naturally in the environment due to bacterial activity.
They must meet strict standards such as they must break down biologically to form CO2, H2O and nothing else.