production and uses of amines
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
give 3 ways of producing amines and state which type of amine they can produce:
reaction of XS NH3 w/ XS haloalkanes - aliphatic
reduction of nitriles (C≡N) - aliphatic
reduction of nitro compounds (NO2) - aromatic
give the general eqn for the reaction of NH3 w/ haloalkanes to form a 1o amine:
(where R = alkyl group and X = halogen)
R-X + 2NH3 → R-NH2 + NH4X
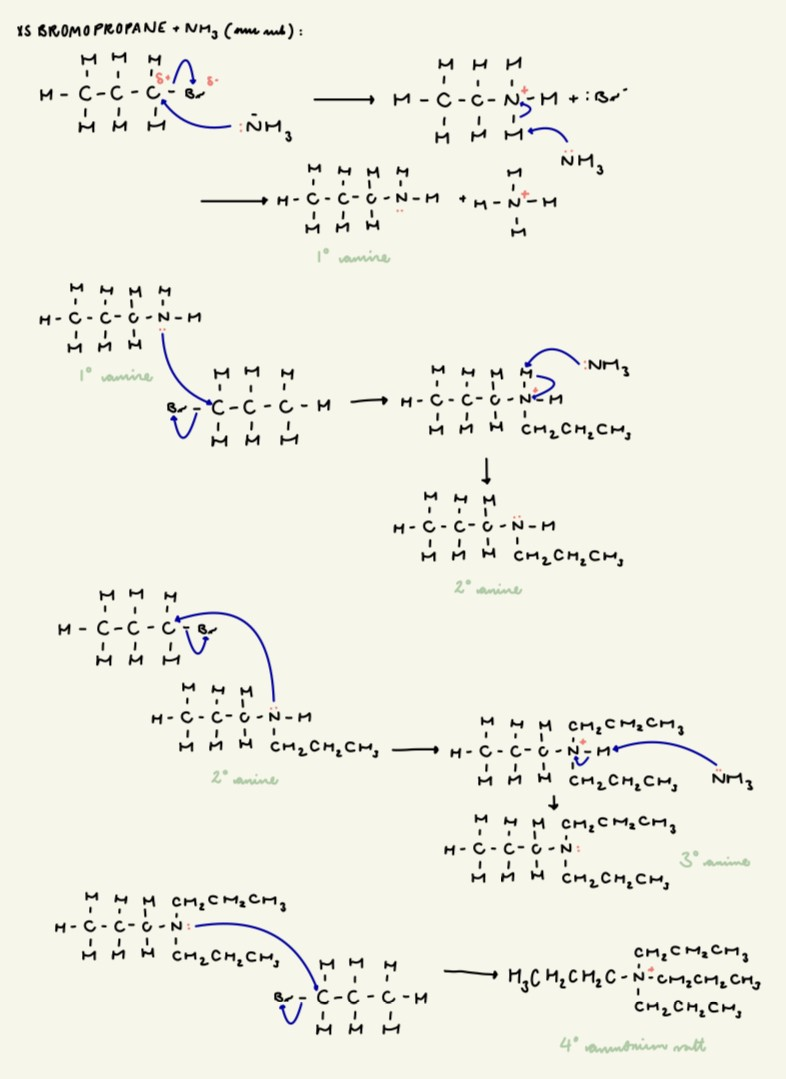
give an issue with using the reaction between NH3 and XS haloalkanes to make primary amines:
products undergo further substitution
this results in a mixture of products (primary, secondary, tertiary and quaternary ammonium salts)
outline the mechanism for the reaction between XS 1-bromopropane and (sufficient) ammonia:
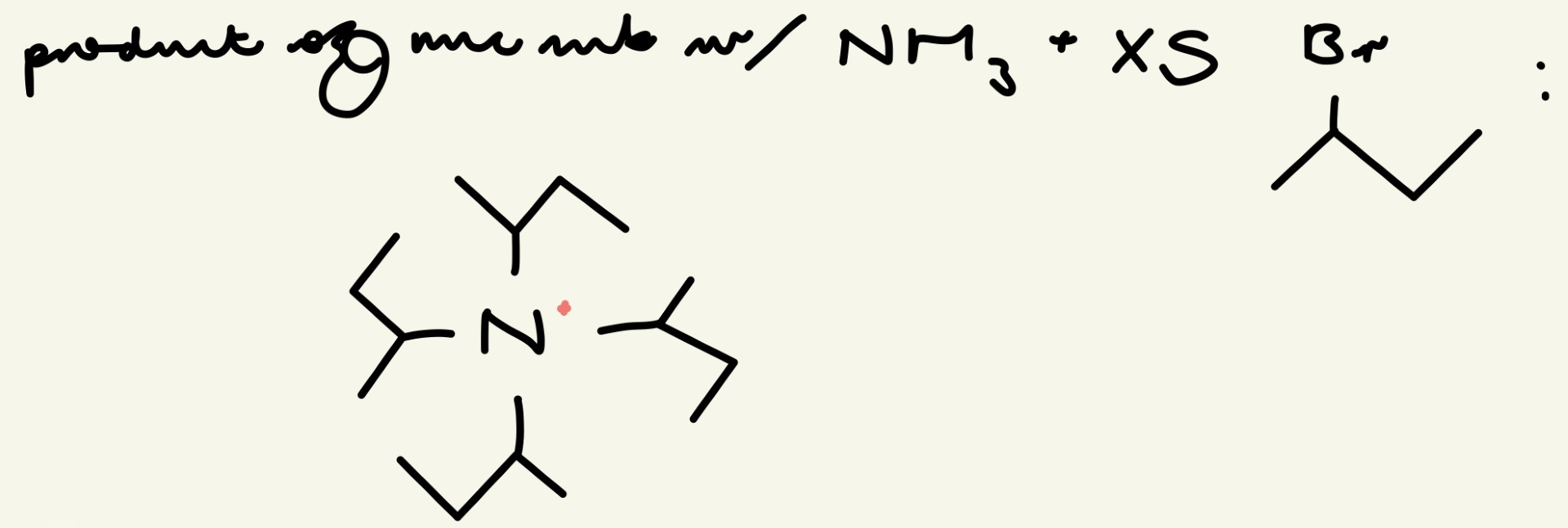
give the final product of a nuc sub reaction w/ NH3 and XS 2-bromopropane:
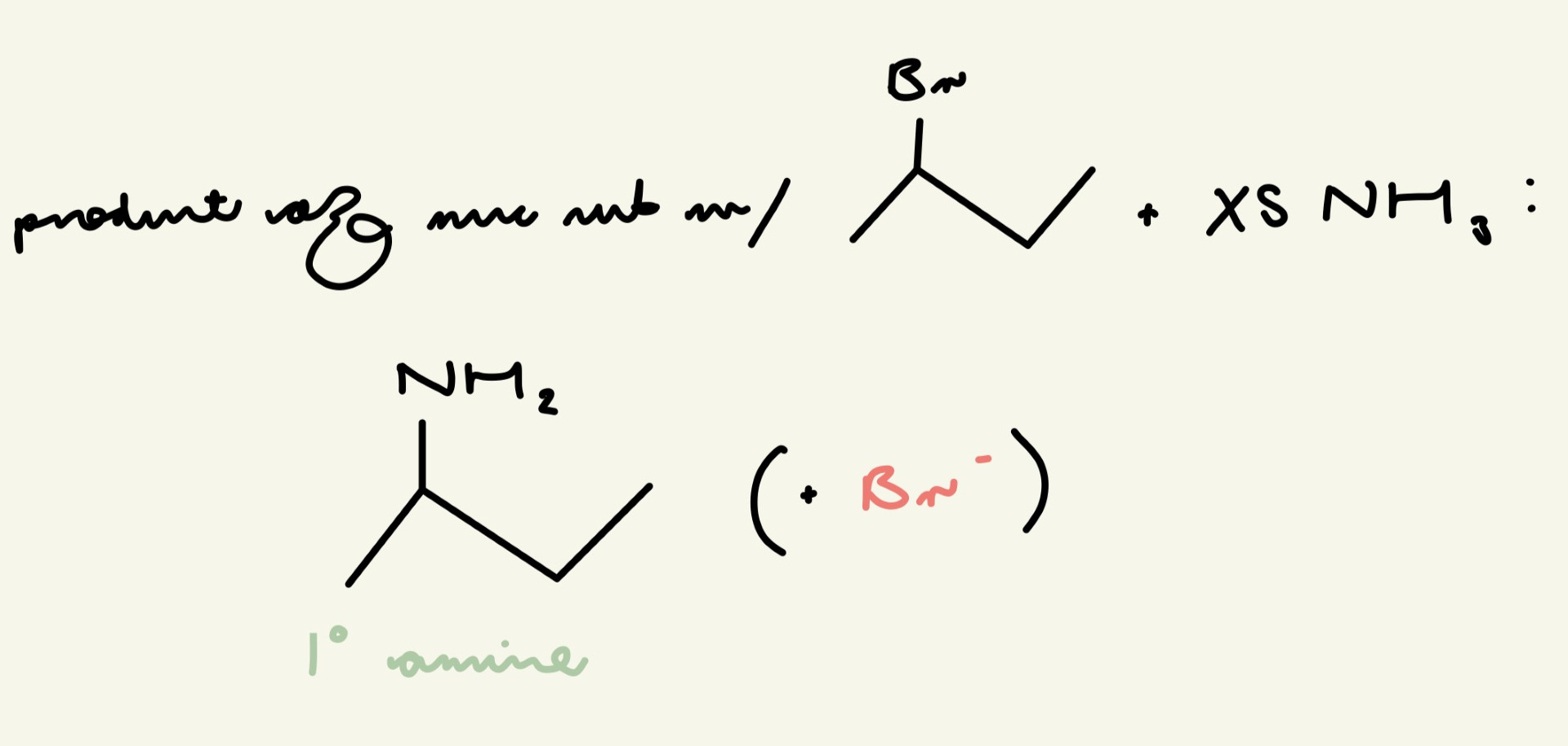
give the final product of a nuc sub reaction w/ 2-bromopropane and XS NH3:
outline the mechanism and eqns for the reaction between bromomethane and potassium nitrile (reduction of nitriles):
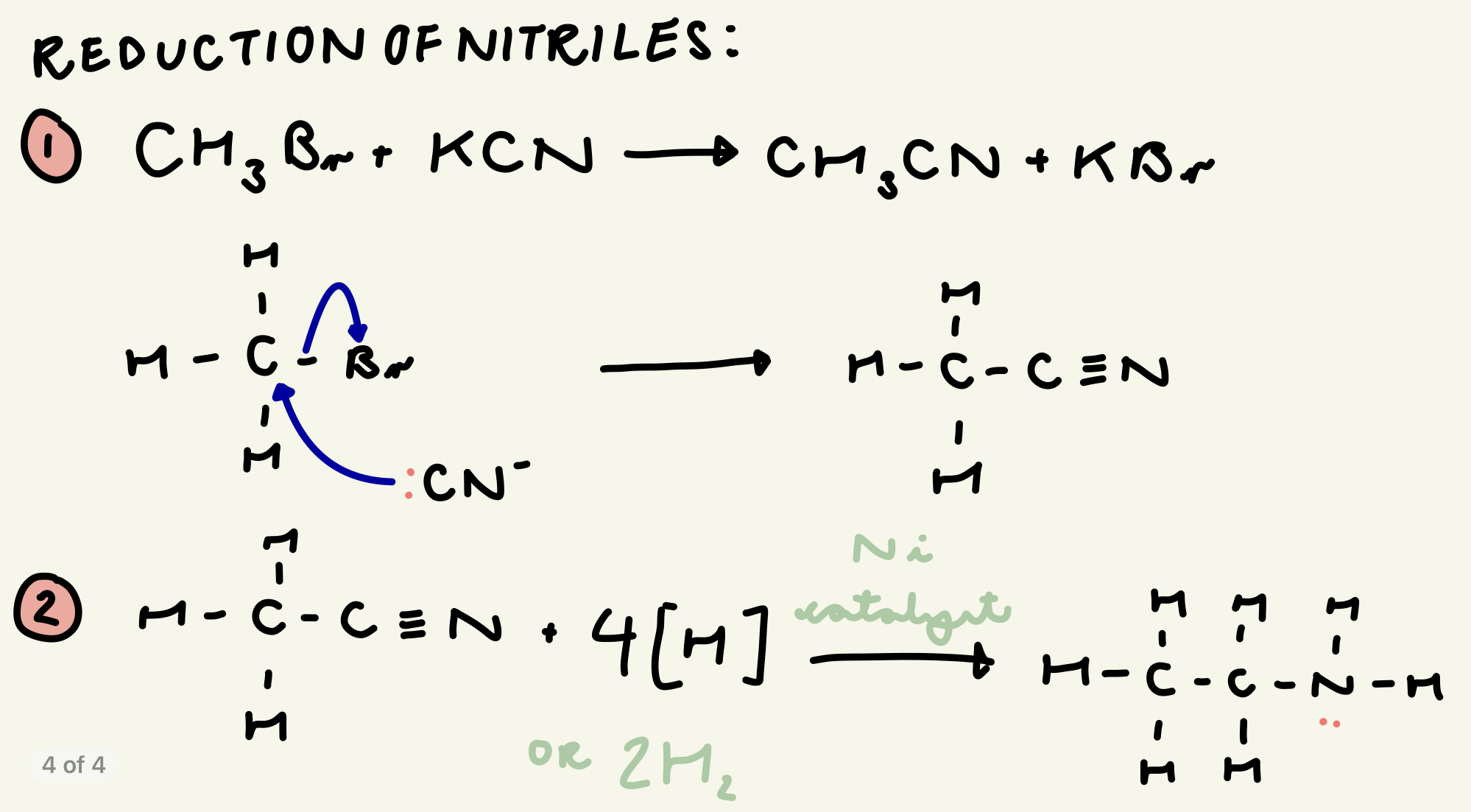
which catalyst catalyses the reduction of nitriles?
nickel (Ni)
why is reduction of nitriles the preferred method for making aliphatic 1o amines?
only one amine formed
increases C chain length - useful
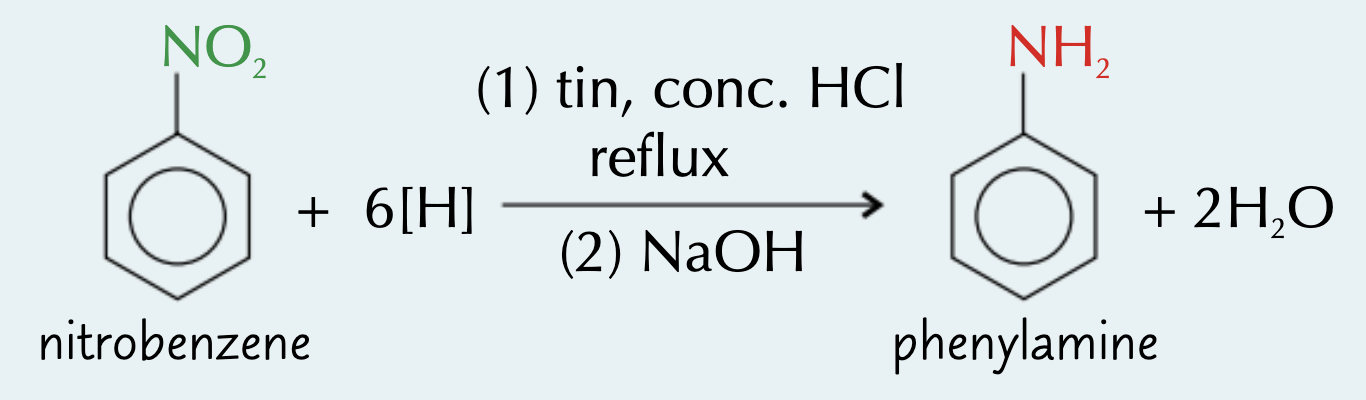
describe the process of making aromatic amines:
reduction of nitro compounds:
heat nitro compound, tin metal and conc HCl under reflux
(add NaOH solution)
give the eqn for the reaction between nitrobenzene and hydrogen (reduction of nitro compounds):
give 2 uses for quaternary ammonium salts:
cationic surfactants
fabric softener as many fabrics have a -ve charge on surface
give one use of aromatic amines:
used in the manufacture of dyes