Chemistry, bonding shapes of molecules simple molecular covalent
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
2 total pairs
Linear
what angle does línea have
180
What does linear look like
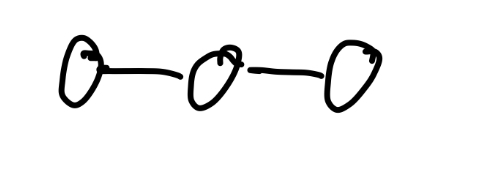
3 total pairs
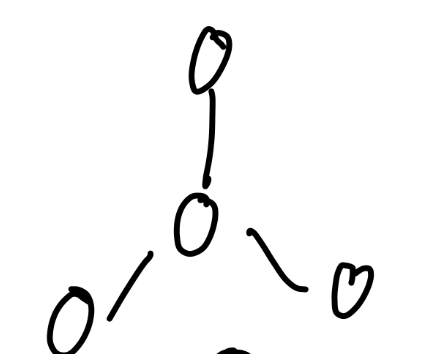
Trigonal planar
What angle is trigonal planar
120
Trigonal planar
4 total pairs
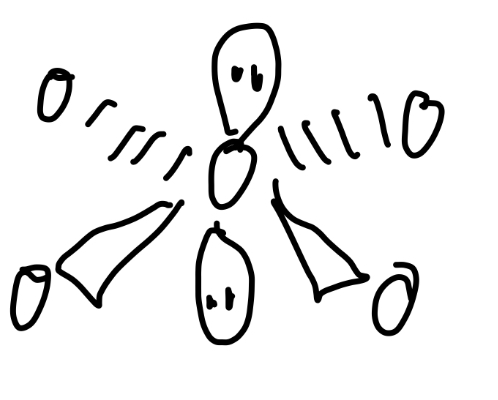
Tetrahedral, triangular bypyramidal, v-shaped
4 bond pairs
Tetrahedral
Angle of tetrahedral
109.5
Tetrahedral
2 bond pairs, 2 lone pairs
V-shaped
Angle of v-shaped
104.5
3 bond pairs, 1 lone pair
Triangular pyramidal
Angle of triagonal pyramidal
107°
Triagonal pyramidal
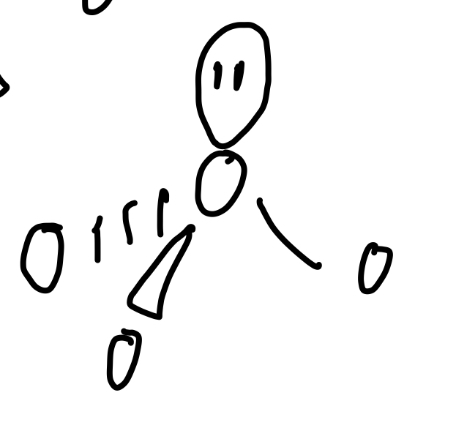
V-shaped
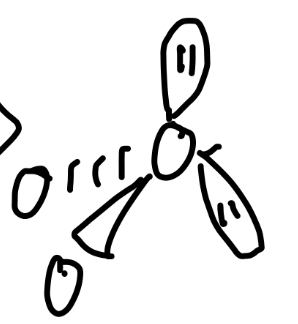
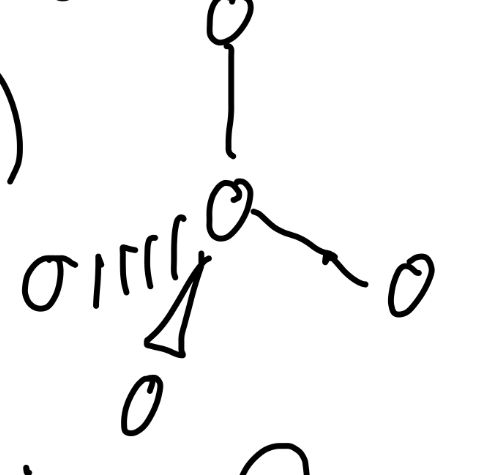
5 total pairs
Trigonal bypyramidal, t-shaped
5 bond pairs
trigonal bypyramidal
Angle of trigonal bypyramidal
120° and 90°
Trigonal bypyramidal
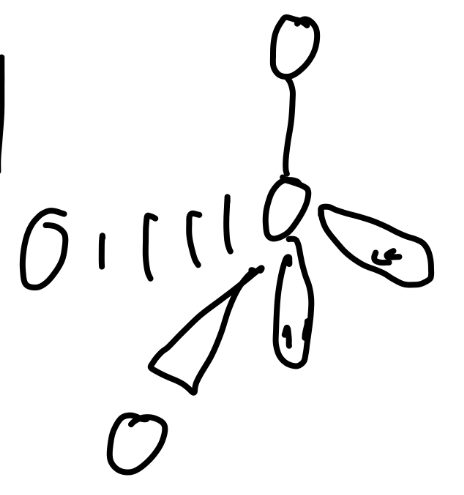
3 bond pairs, 2 lone pairs
T-shaped
Angle of t-shaped
90°
T-shaped
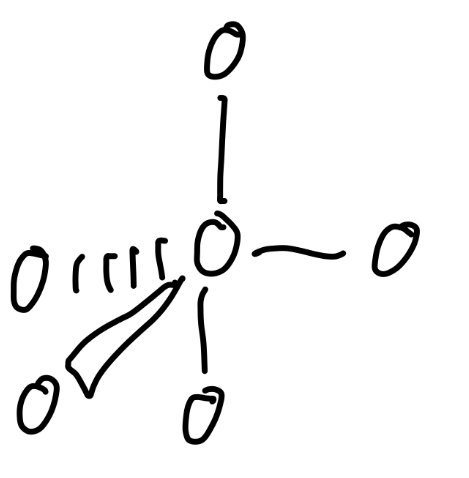
6 total pairs
Octahedral, square planar
6 bond pairs
Octahedral
Angle of octahedral
90°
Octahedral
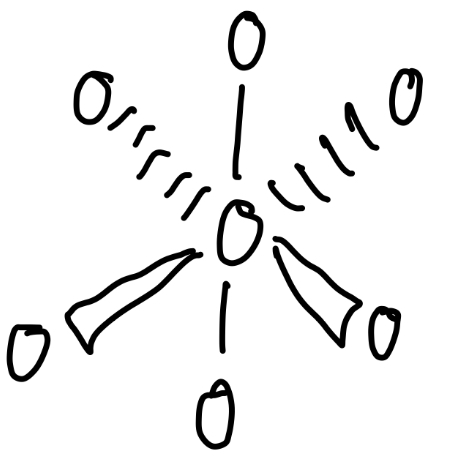
4 bond pairs, 2 lone pairs
Square planar
Angle of square-planar
90°
Square planar