BIOL 2052 - Glia
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
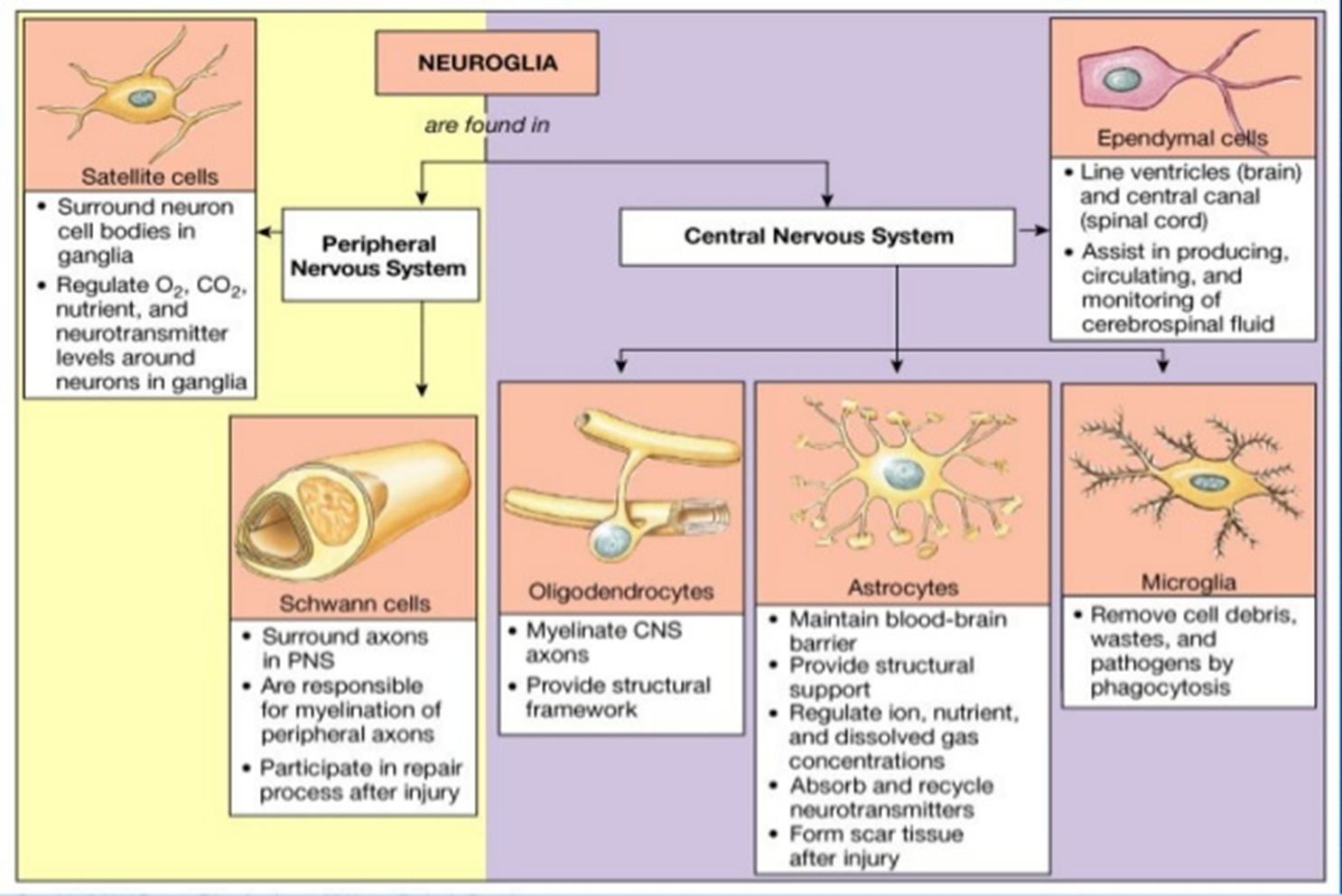
defining glia
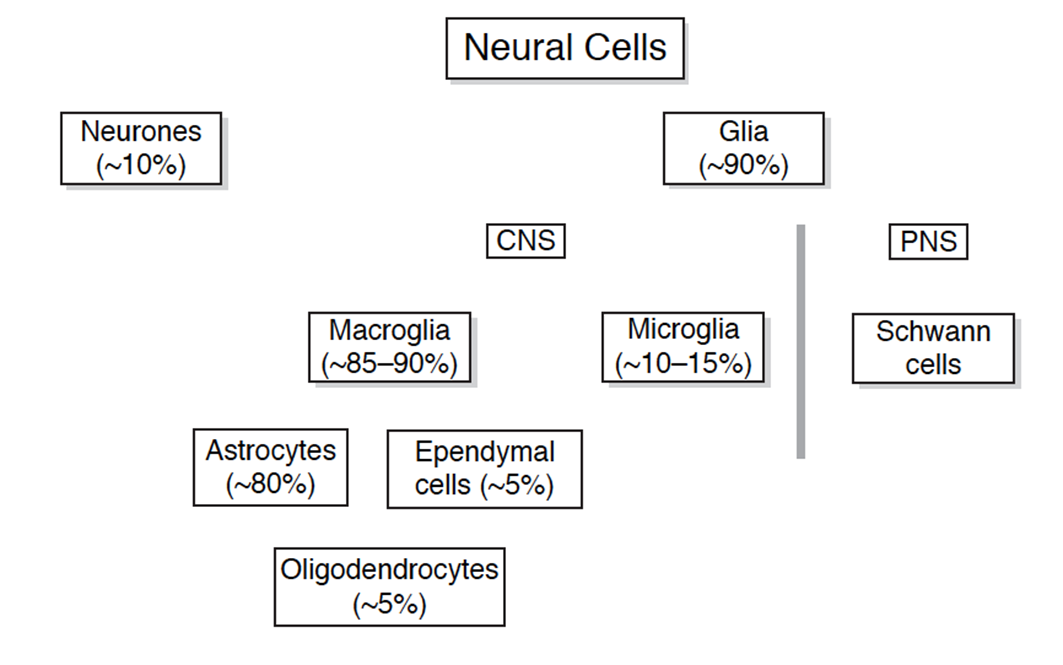
macroglia - a group of cells - anything that is a glia but is not a microglia
glial cells do not carry synaptic function but essential for function of NS
around 90% of the cells in the brain are glia
without glia the neurons wont work
of the 90% divided into CNS and PNS
PNS - schwann cells
CNS - macroglia (85-90%) and microglia (10-15%)
macroglia can then further be subdivided into ependymal cells (5%), oligodenrocytes (5%) , astrocytes (80%)
function of glia
MAIN FUNCTIONS
provide physical support
supply nutrients and oxygen to neurons
to insulate one neuron from another and faciliate synaptic communication
destroy/remove cell debris
OTHER SPECIFIC FUNCTIONS
critical development roles
glial migration
growth/direction of axon/dendrites
modulate synpatic transmission
fundemental role in disease/degeneration —> as you get more complex brain function you require more glia and increased size of glia with more glia per neurons
discovery of glia
Rudolf Virchow - 1956 first discovered glia (glue) didnt think they were functionally relevant
Deiters then begins to describe them - lack of axons
Deiters was the first to suggest ectodermic origin - same origin as neurons
andriezeen then began to classify types of glia
ectodermal/fibrous glia in white matter
mesoblastic protoplasmic glia in grey matter
Cajal then proved that these 2 glia both were ectodermically derived but suggested 3 types of glia —> later proposed that there were 4 types of glia:
protoplasmic
neuroglia
mesoblastic glia
interfasicular glia (oligodendrocytes)
many functions recognised
plasticity
electrical insulation
pathological role
evidence for glial origin
radial glia have their body in the ventricular surface
act as a scaffold for migration of glia
but these cells persist in adults, they can give rise to astrocytes, microglia, oligodendrocytes
development of the brain
synaptic pruning and myelination can take up to 20 years (critical for learning)
Before this neurogenesis also important
glia become important about halfway through embryonic development when neurons start to connect to each other
microglia are the exception - come from an extra neuronal origin and remain in the brain as the BBB closes
O2A progenitor
stem cells develop into O2A progenitor cells - cells which have already comitted to either development of astrocytes or oligodendrocytes
O2A progenitor replicates into astrocytes to start but once we have made the astrocytes it becomes an NG2 cell (expressing NG2) which wil form oligodendrocytes
NG2 cells dormant but can be reactivated if theres a need for astrocytes (E.g: demyelinary disorder
NOTE: O2A cells and NG2 cells are synonymous, the class of cells is O2A but are often called NG2 cells as they express NG2
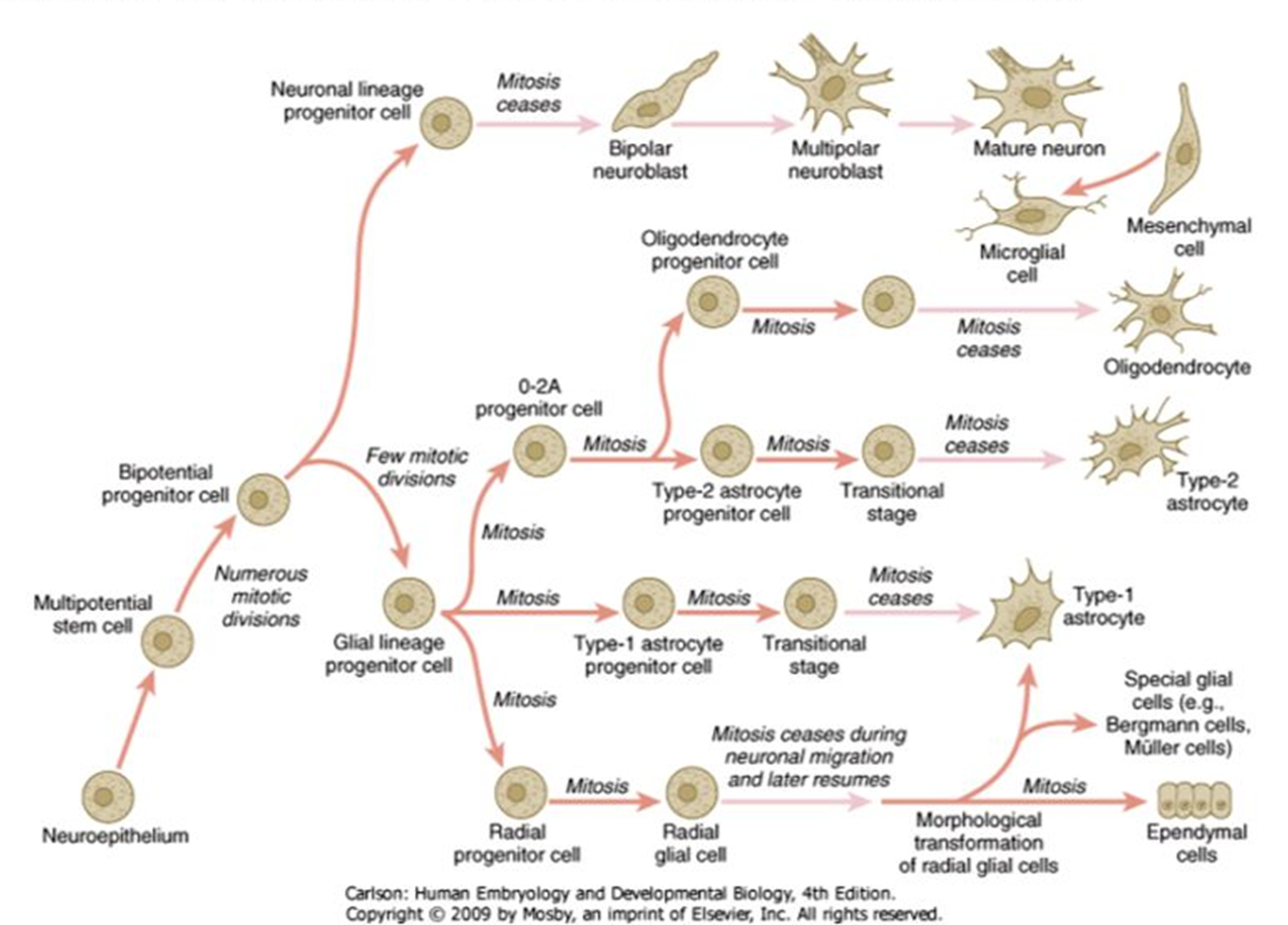
maturation of oligodendrocytes
Neural stem cells
Multipotent cells in the ventricular zone.
Can give rise to neurons, astrocytes, and oligodendrocyte lineage cells.
Oligodendrocyte progenitor cells (OPCs)
express Ng2 at this stage
sometimes synonymous with O2A
O2A
subpopulation of OPCs
express NG2 and A2B5
can become mature oligodendrocytes or type 2 astrocytes
pre oligodendrocytes
start to express O4 but no myelin proteins
less proliferative, more committed to differentiation
immature oligodenrocyte
no expressed NG2, expression of O4
begin extending processes
express low levels of MBP and MOG but are not yet fully myelinating
mature oligodendrocytes
express proteins that occur in myelin (PLC, MAG and MBP)
transcription factors define the transition (oligodendrocyte precursors express high levels fo notch 1 and prox 1)
lost notch 1 and increased prox 1 causes progression to next cell stage
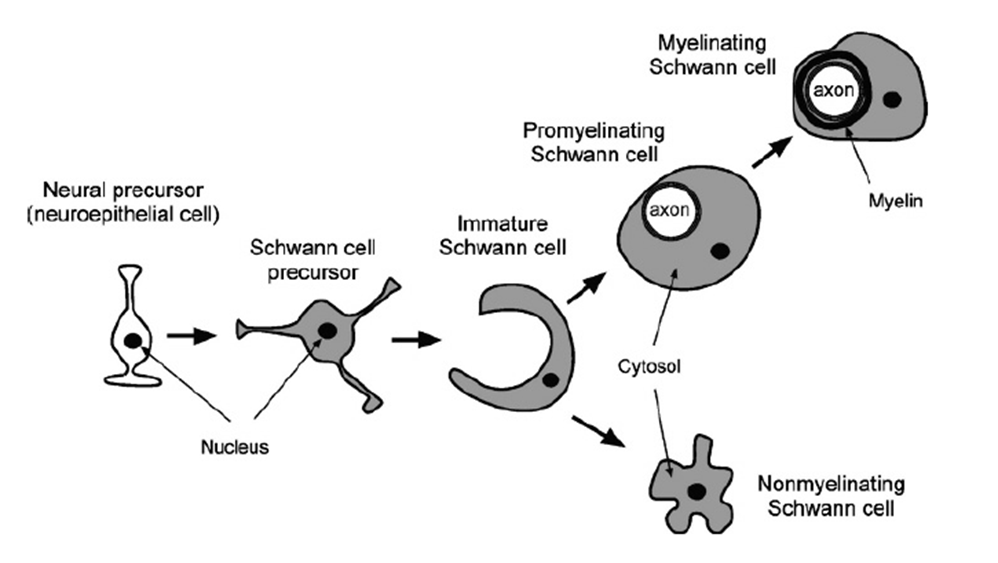
schwann cells
highly related to oligodendrocytes
similar stepwise development
neural precursor —> schwann cell precursor —> immature schwann cell which forms either
promyelinating schwann cell —> myelinating schwann cell
non myelinating schwann cells (provide support)
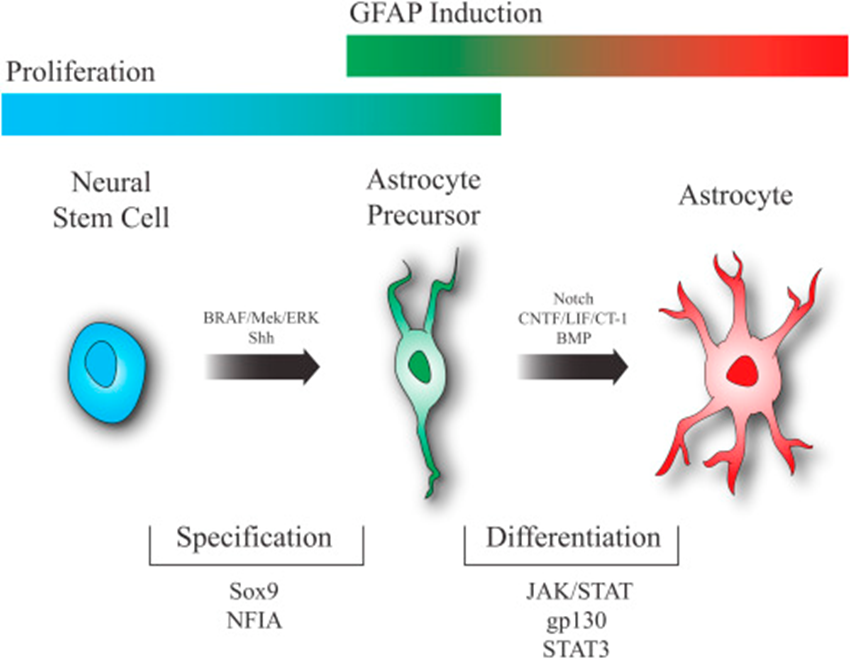
astrocyte
neural stem cell —> astrocyte precursor —> astrocyte
stages of astrocyte lineage poorly defined and lack stage specific markers
do express certain proteins at certain development points (E.g: GFAP is an intermediate filament required for the mature structure)
sox 9 is essential for neural stem cell to progress to astrocyte progenitor
activation of Jak/stat essential for astrocyte precursor to develop to astrocytes
potential for multiple types of astrocyte
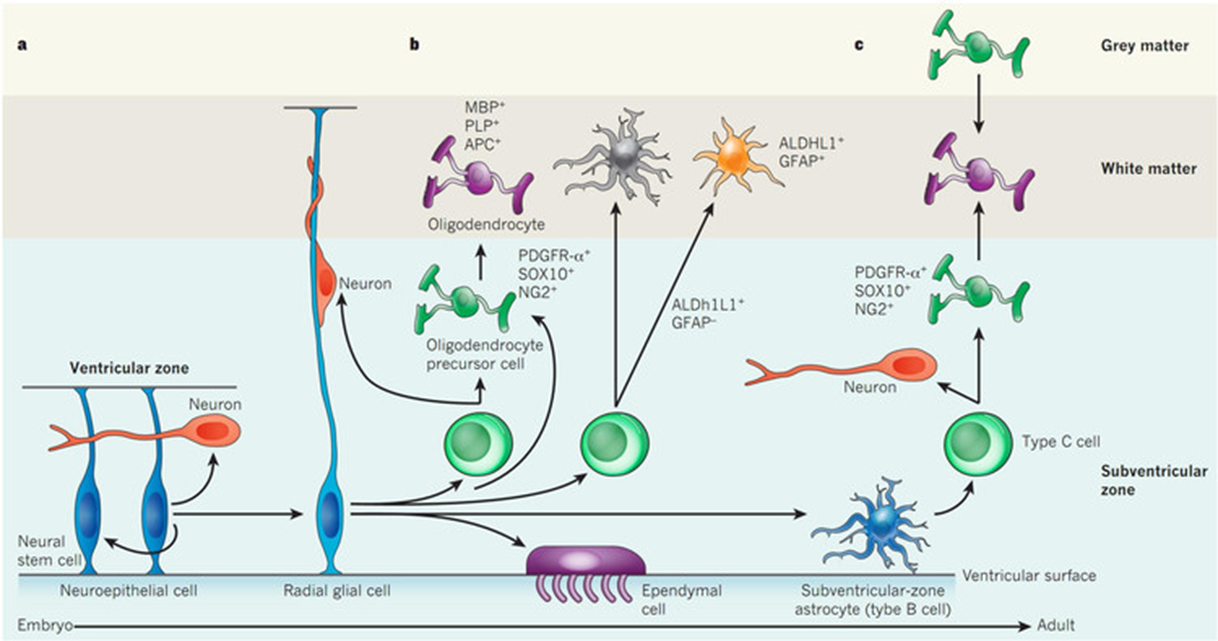
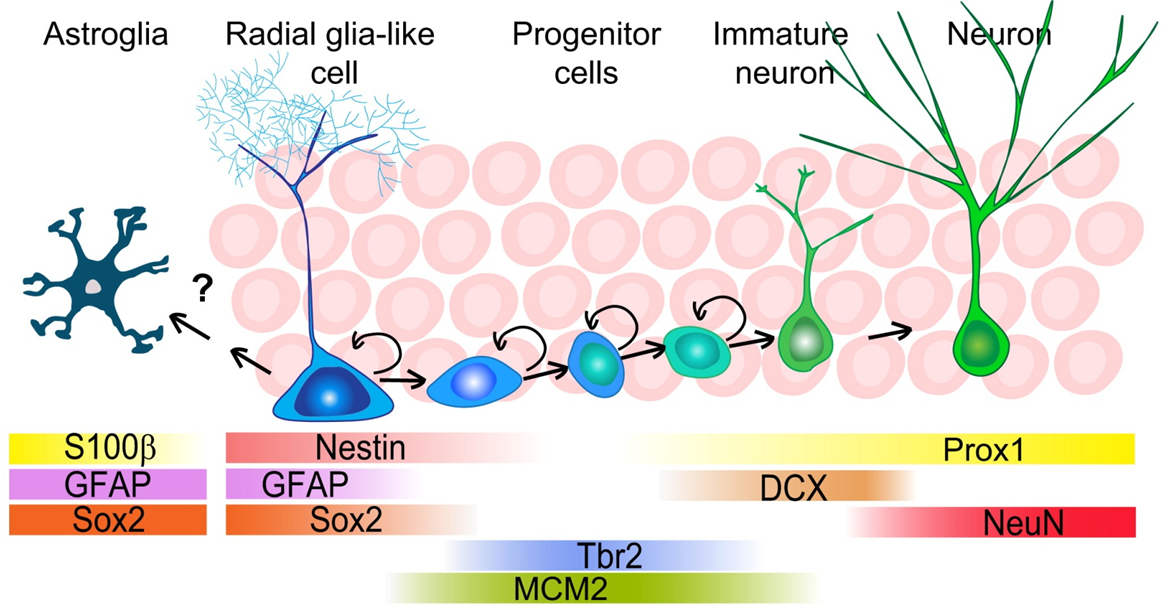
generation of new glia
radioglia common precursor for glia/neural cells
cajal had the hypothesis that once the brain was deveoped no new cells formed - turns out to be wrong, new neurons constantly produced in:
dentate gyrus
ventricular zone
new neuronal cells important for moving to the olfactory bulb and formation of memories in the hippocampus
default state for radioglia to generate astrocytes when they dont generate more neuronal cells
if we can embedd TFs can we repolarise specialised cells to earlier progenitor cells —> this means that the generation of these cells likely to be from the same cell
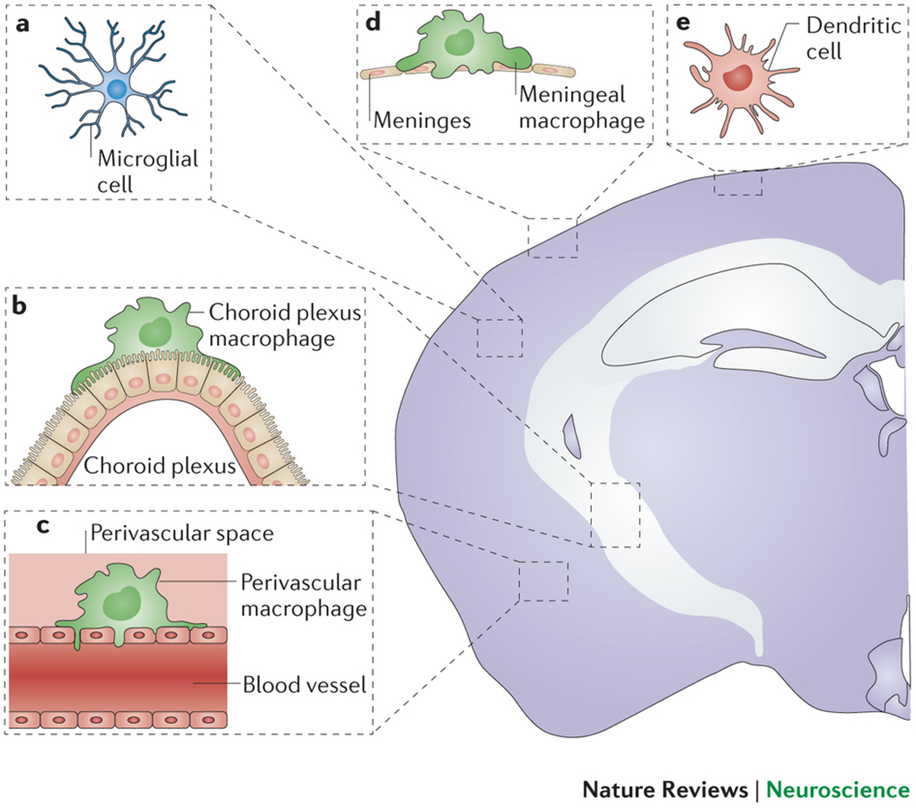
microglia
are actually macrophages —> not macrophage like
one of the immune cells in the brain —> lots of immune cells in the membranes surrounding the brain and within the parenchyma but not much known
immune census of the brain: many cell types clustered within regions of the brain but many sub populations within these populations
macrophage subpopulation:
choroid plexus macrophage
perivascular macrophage
meningeal macrophage
even just residing in the brain gives some kind of similarity to any kind of macrophages
we can predict what functions these macrophages might have
basic characteristics of microglia
highly ramified → tile the brain parenchyma in a mosaic like distribution (no overlap)
projections move, cell body doesnt
difference between white and grey matter microglia:
white: highly polarised
grey: more ramified
different densities between regions
equipped with immune sensors to return the brain to homeostatic state
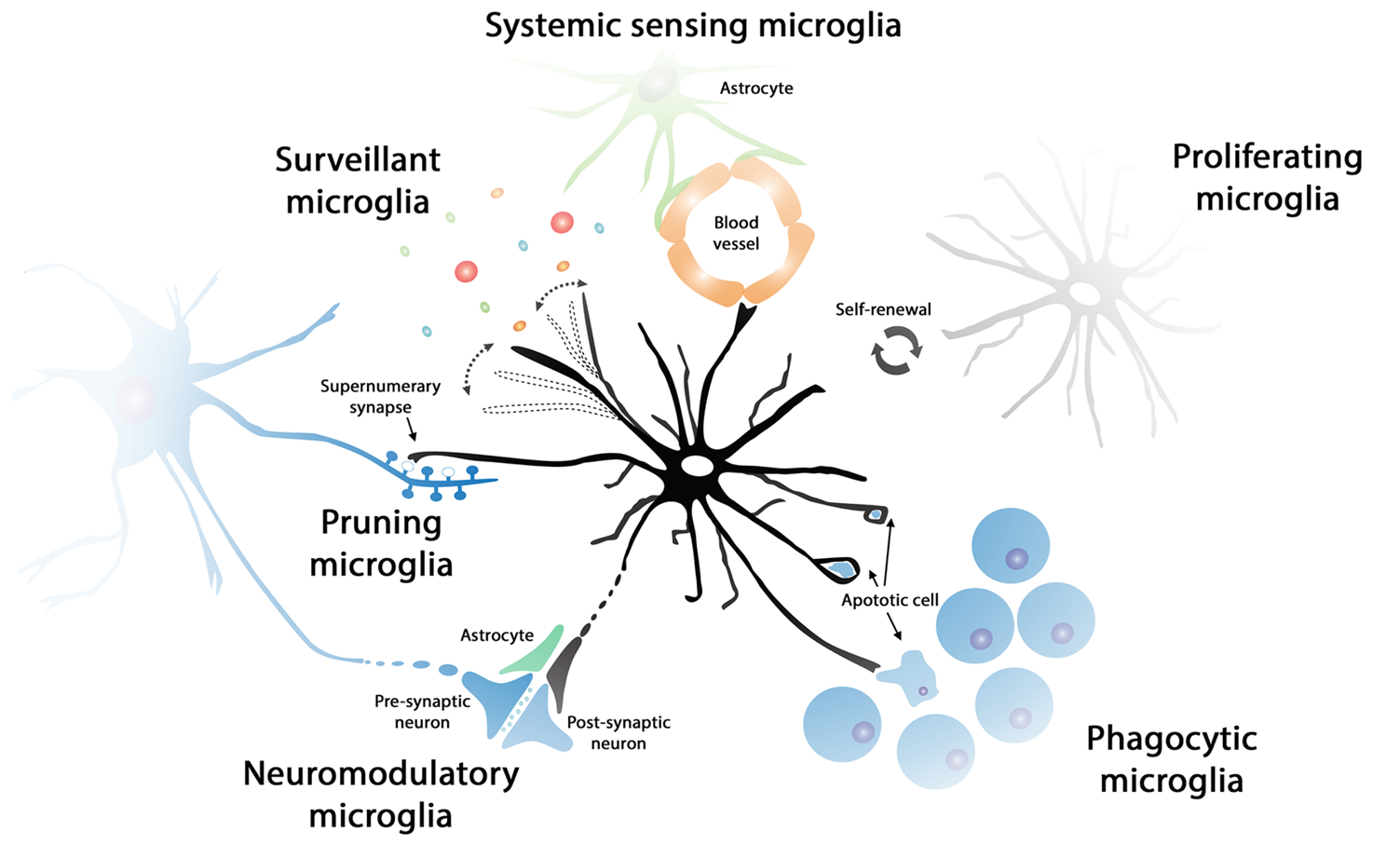
systemic sensing microglia
can phagocytose synaptic elements, dead cells etc
postulated that microglia can prune synapses which need to be removed and can modulate synaptic activity
good at sensing inflammation
communicate with the rest of the immune system
discovery of microglia
discovered with nissal staining
first named based off their less round nucleus (stabenzellen)
observed with a shape change in animals with rabies
showed that perivascular microglia derived from bone marrow
development of microglia
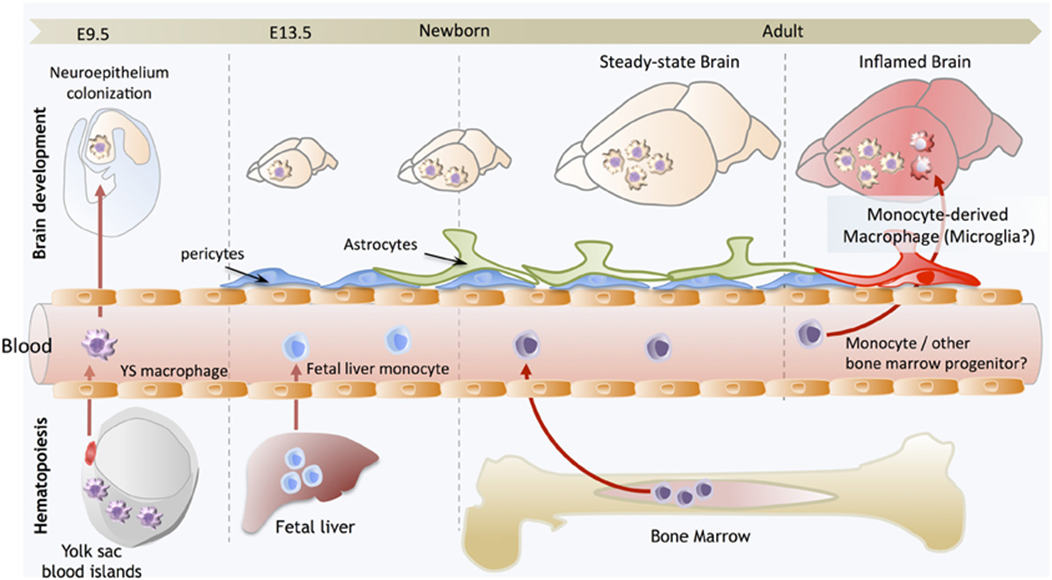
erythromyeloid progenitors derived from yolk sac (not part of the embryo but has stem cells) give rise to all macrophage population
colonise the brain (whilst the BBB is open)
after closure we cant get cell migration to the brain
foetal liver main heamopoetic organ giving rise to cells
they eminate from the liver, colonise the other organs and develop macrophage populations
in the brain microglia are sustained by cell renewal, whilst in other organs macrophages can eb replaced by bone marrow stem cells
in cases of damage to BBB some macrophages might enter the brain and differentiate into microglia like cells
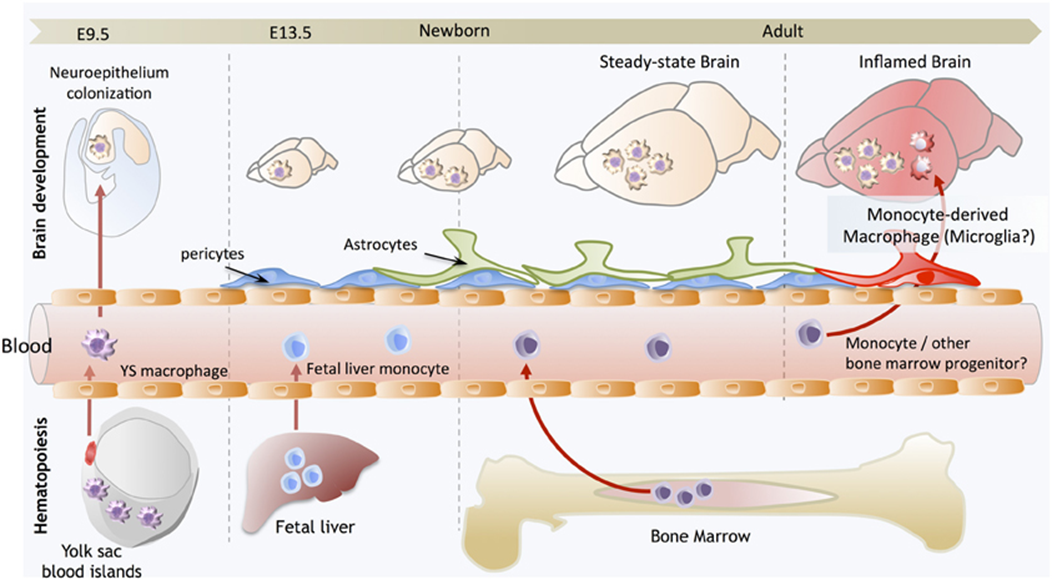
microglia lineage
EMP (erythromyeloid progenitor cells) colonise the brain
differentiate into premacrophage (common to other macrophage cell types)
premacrophage (A1CD45+CX3CR1 is low, so is F4/80) —> premicroglial cell (A2CD45+, CX3CR1 high, F4/80 high)
this commits the cells to microglia
conversion of A1-A2 is controlled by the upregulation of TF PU.1 (genetic factors which do not depend on the environment)
conversion of A2 —> microglia driven by the environment (surrounded by IL34, TGFbeta which is not found anywhere else)
only when cells start to develop into A2 do they express proteins characteristic of macrophages
transcription and functional diversity
microglia not equal —> diff environment drives diff phenotype (degree of heterogeneity)
genes and TFs which are upregulated in cerebellum that are not upregulated in the striatum and the cortex
degree of similarity in the hippocampus and cerebellar cortex but still distinct
cells provide info about energy metabolism, immune response etc of tissue
distance of dissimilarity with other macrophage is huge but equally all macrophages in the brain are not similar
populations of microglia associated with injury, development, aging, postnatal development etc.
there is heterogeneity of macrophages linked to environmental/developmental factors
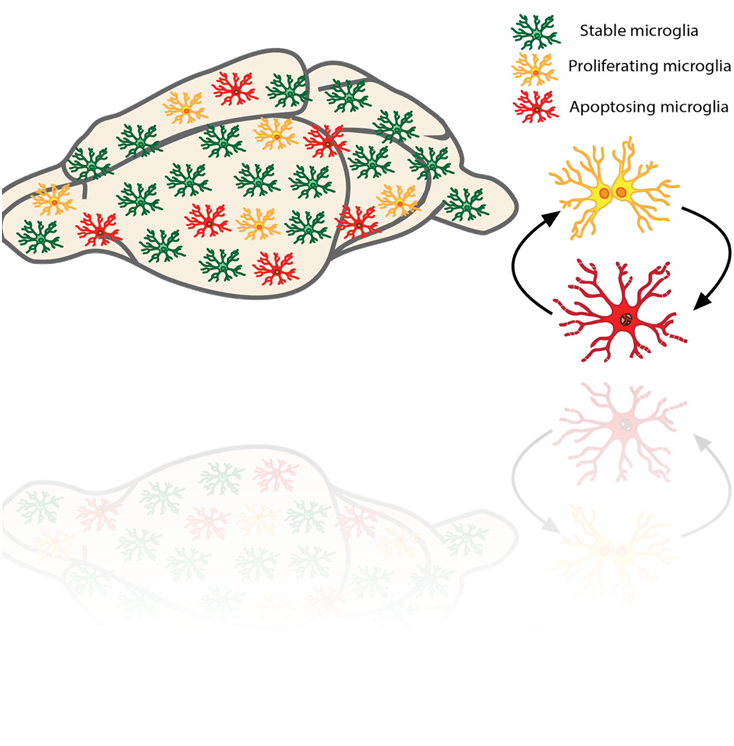
microglial density is not affected by aging —> must either be very long lived or proliferating - turns out to be cycling proven by:
can dose cells with Brdu and doesnt affect cell cycling
allows us to know which cells are replicating at a certain time
if we check at different periods we can compare rate of apoptosis compared to rate of growth
microglia proliferate at a surprisingly high rate - BIM dependent apoptosis balances microglial proliferation and apoptosis allowing constant turnover (6.6 times in mouse lifetime)
role of astrocytes
neurogenesis in the adult brain
neuronal guidance in developmental rols of radioglia
regulation of synaptogenesis and synaptic maturation (TGF beta can induce formation of excitatory and inhibitory synapses)
structural —> responsible for defining and connecting domains that include neurons, synaptic vessels, blood vessels
communicate through gap junctions
make up the BBB
tripartate synapse
majority of synapses have a third astrocyte component (60%)
pre and post synaptic neuron sealed by astrocyte neurotransmitter in contact with astrocyte
80% of large perforated synapses are enwrapped by astrocytes
in the cerebellum, innervation of the purkinje cells with bergmann cells (astrocyes of the cerebellum) each enwrapping 2000-6000 synaptic contacts
possibility to integrate and modulate activity as recieve inputs from hundreds of cells
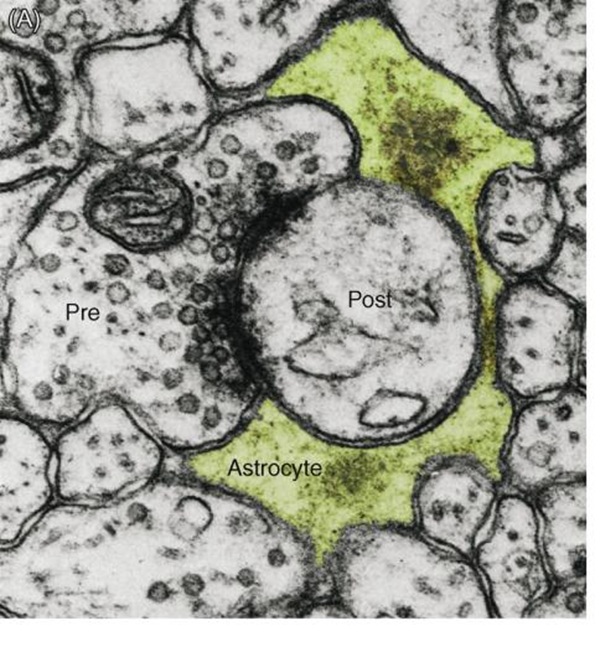
evidence for tripartate synapses
astrocytes are excitable, can produce a transient change in IC Ca2+ conc through release from ER stores (astrocytes get excited by the release of neurotransmitters which can be sensed by changes in extracellular fluid composition)
lots of astrocytes respond shortly after the stimulation of an axon
astrocytes communicate bidirectionally:
able to detect neurotransmitters and other signal released from the neurons at the synapse and can release their own neurotransmitters or gliotransmitters that are capable of exciting neurons (regulated by IC calcium levels)
all astrocytes communicate via gap junctions - can illicit a response in astrocyte not in contact with the original syanpase - must be a non neuronal way of communicating
works via glutamate signalling
astrocytes can also regulate the concentration of extracellular ions such as potassion which can directly influence neuronal activity.
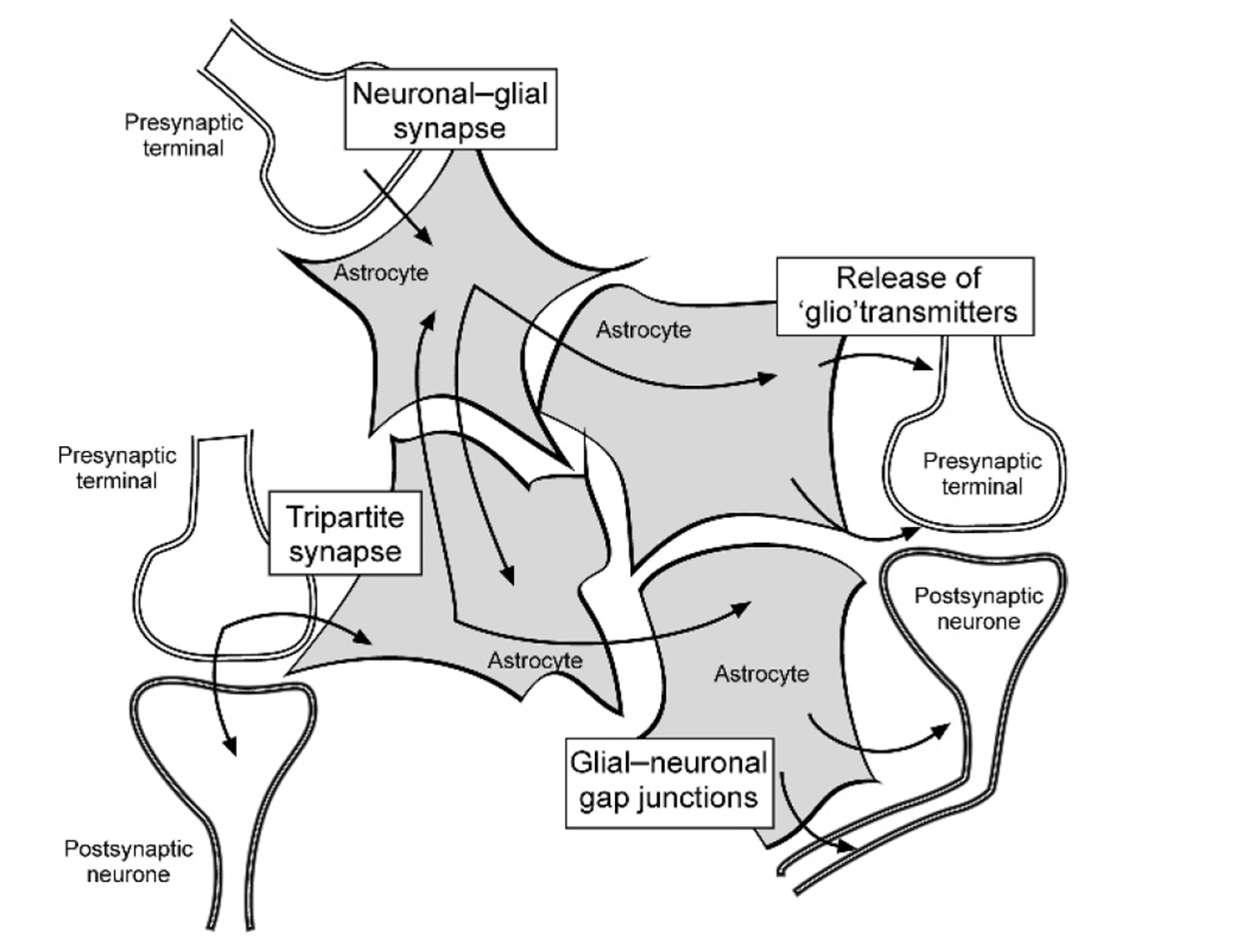
glutamate signalling
astricytes clear glutamine from the synaptic cleft
glutamine is a target for kainate receptors, metabolic receptors, glutamate receptors and NMDA receptors
the ability of astrocytes taking up the Glu prevents ot from interacting with neurons and modulates activity
also allows astrocytes to synchronise neurons in the next cycle of activation (synchranous depolarisation)
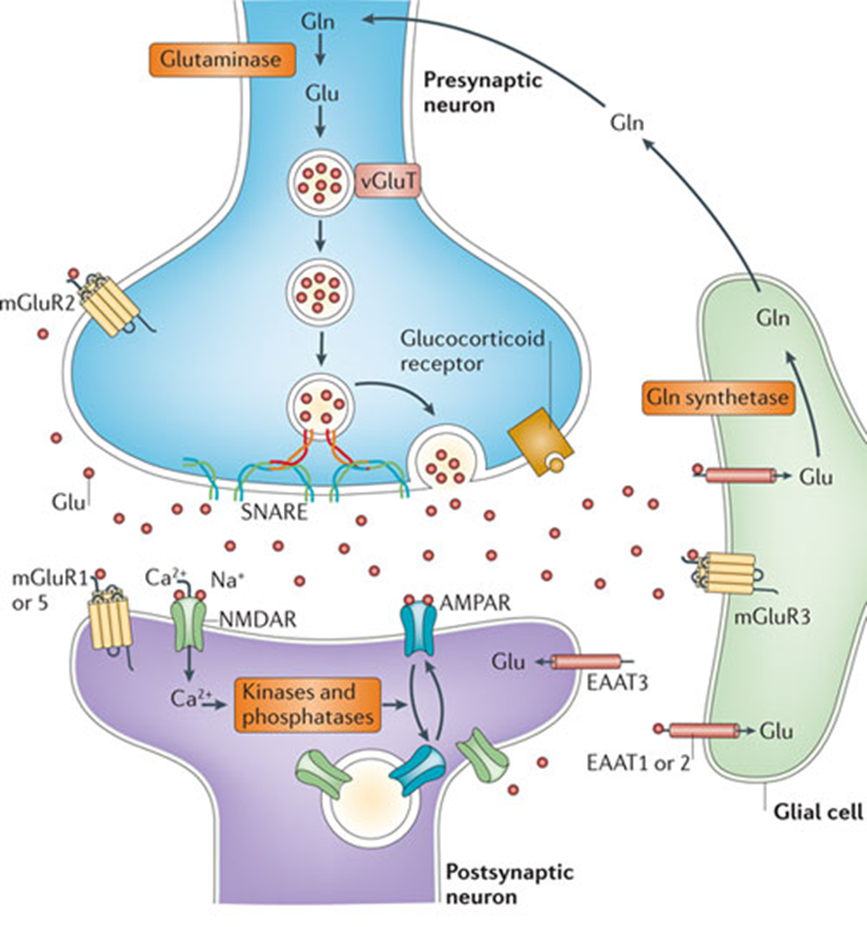
other giotransmitters
ATP targets P2X receptors, P2Y receptors (present on astrocyte and neuronal membrane
ATP produced in astrocyte which can signal to neighbouring astrocyte and either cause it to produce more ATP, Ca2+ etc or it can signal to neuron and tell it to release more or less gluatamate
can also modulate AMPA receptors on post synaptic membrane
ATP release mechanism not well understood - known to be connected to Ca2+ waves, likely related to SNARE proteins
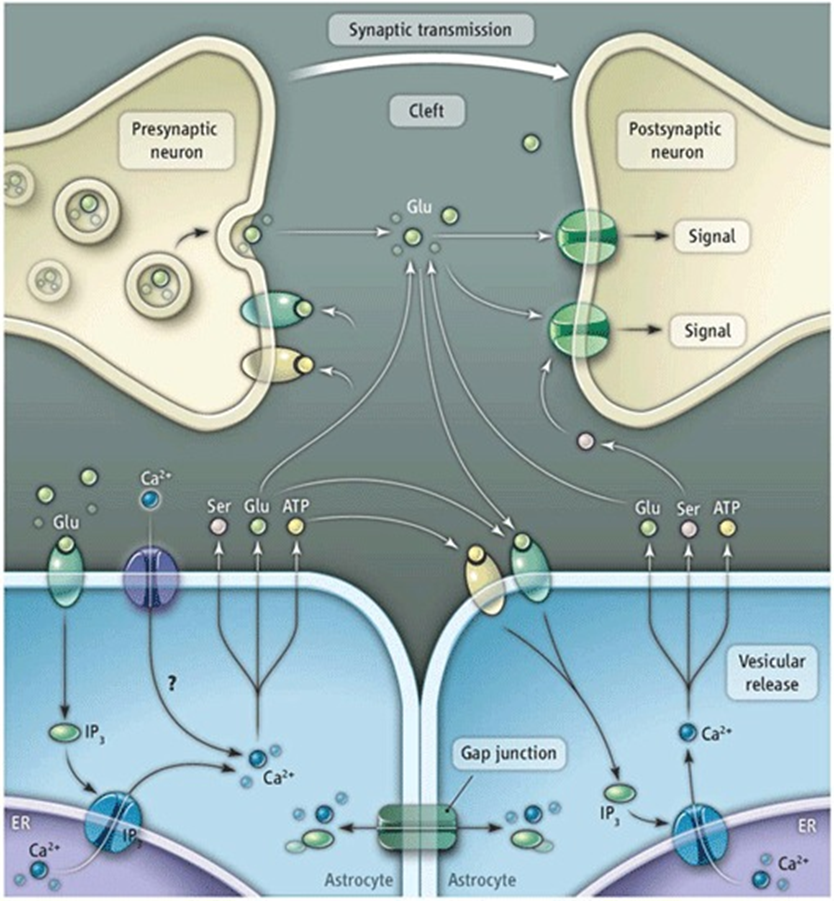
distal regulation: example
astrocytes of teh hippocampal startum oriens form tripartate synapses with axonal projections from the alveolus
alveolus projections can be either glutameric or cholinergic synapses with the stratum oriens but teh astrocytes of this region respond with changes in Ca2+ conc (only to cholinergic activation of alveolus projections)
not just a passive response, actively responding
astrocytes produce more glutamate at cholinergic synapse due to cholinergic signalling —> keep neurons signalling regardless of neurons activity
integration and modulation of activity
the hippocampus striatum oriens astrocytes which respond to synaptic activity from the glutameric neurons originating in the chaffer collateral and cholinergic neurons originating in the alvus
they produce changes in Ca2+ conc that are non linear with synaptic input
additionally, the same stimuli are capable of producing either a potentiates Ca2+ response at low frequencies of stimulation or a depressed Ca2+ conc at high frequencies of stimulation
the blood brain barrier
barrier between the intercerebral blood vessels and the brain parenchyma
endothelial cells closed by tight junctions
every peice of capillary wrapped by astrocyte end foot
2 components: bood and parenchymal compartment
present everywhere throughout the brain except circumventricular organs, neurophyphoris, pineal gland, subfornical organ and neuroendocrine signalling (need to be able to check blood content fast)
every solute must pass through endotheial cells: selectively permeable to essential nutreints to enter and metabolites to have
specific trasporters at the endothelial cells include:
amino acid transporter
energy dependent ABC transporters which excrete xenobiotics (impermeable to drugs, antibiotics etc)
GLUT1 glucose transporters
ion exchangers
transporters at the astrocyte endfeet include:
glucose transporters, uptake and distribute to neurons
K+ chnnels
water channels
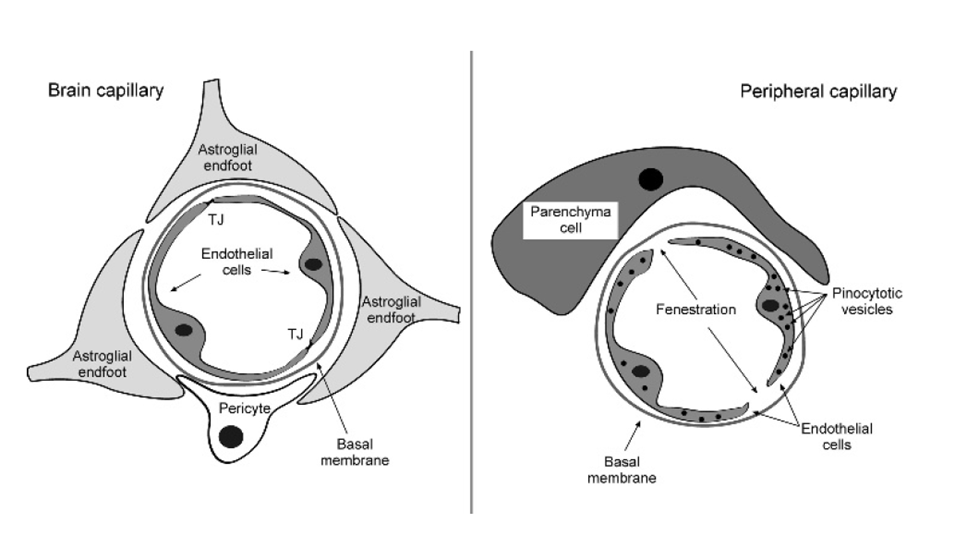
astrocyte and spinal chord injury
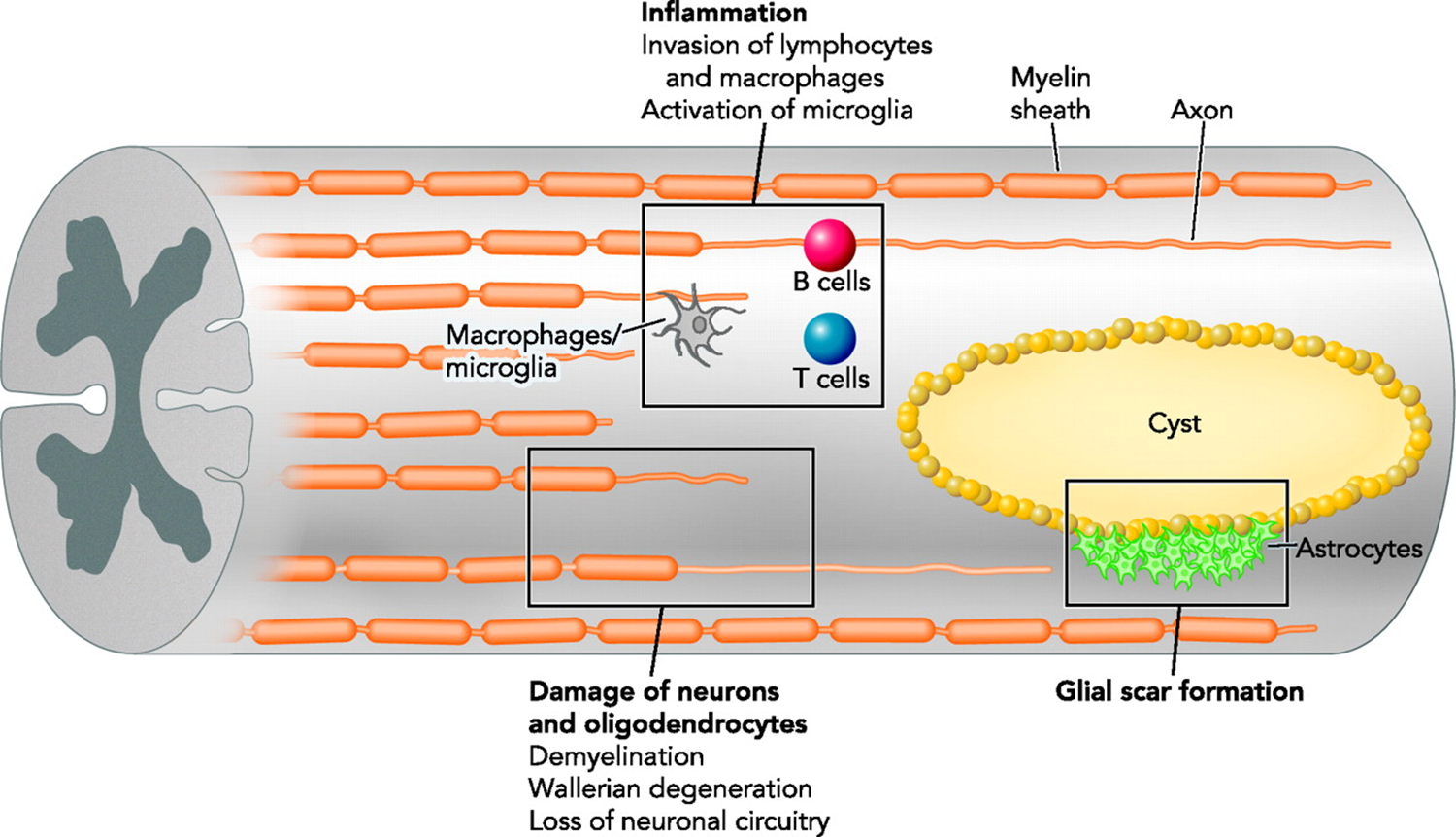
damage to the BBB causes damage to the environment of the brain which the astrictyes react to:
spinal chord injury can causse separation of pre and post synaptic neuron as well as blood components in spinal chord which activates glial cells
astrocytes from glial scar to form a barrier to protect the environment - prevent flow of damaging molecules into the tissue (proliferate and join end feet)
protection from secondary injury
glial scar is hard to remove and remains for a long time - doesnt allow neurons to grow through it
causes complete separation of ascending and descending signals
once all of the dead waste/debris cleared up it forms a cystic cavity surrounded by astroctyes
physical and molecular barriers
astroctyes also secrete growth promoting molecules in attemot to regenerate
growth cone retreats if it comes in contact with astrocytes
inability of cells to cross the barrier not caused by astrocyte but intrinsic failiure of neurons to grow through glial scar
PI3K key pathway for growth cone, hen PTEN activated it inhibits this
when PTEN inhibited the axon is able to grow through glial scar
myelination
oligodendrocytes
all myelination in CNS is done by oligodendrocytes
multiple axons myelinated by OG cell (avg 10/cell)
schwann cell
myelinate in PNS
schwann cells myelinate in a 1:1 ratio
myelination is dependent on axon diameter
lamellae refers to the number of layers of glial cell membrane wrappiing around the axon
radial growth of axons and myelin sheath are interdependent
ratio of axons to myelin lamellae is called the g ratio and it is constant in the CNS and the PNS (1:10)
interdependence of glia axons
the loss of the axon results in degeneration of oligodendrocyte and differentiation in schwann cells
conversely, aons degenerate in absence of support cells (OG and schwann cells)
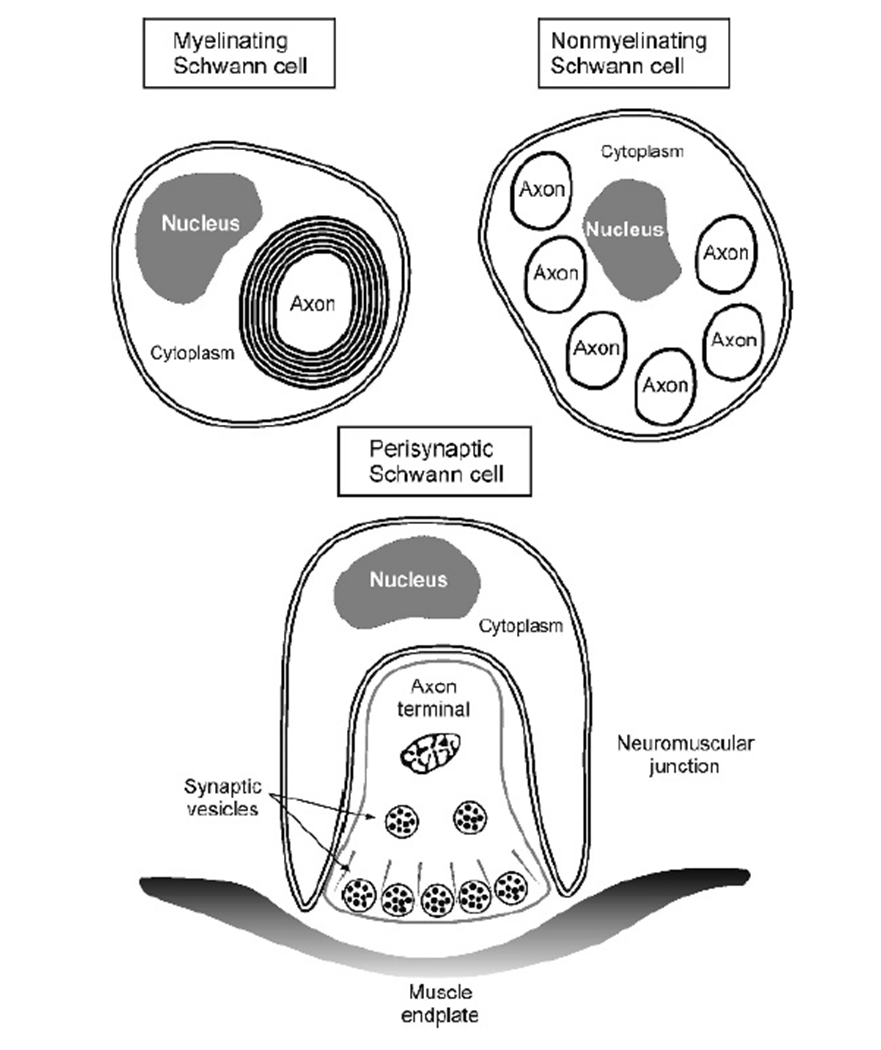
schwann cells
NON MYELINATING SCHWANN CELLS
non myelinating schwann cells usually surround bundles providing trophic support
express L1 and NCAM which are not found on myelinating schwann cells
PERISYNAPTIC SCHWANN CELLS
3rd type of schwann cells are perisynaptic schwann cells (found in the NMJ)
ensheath the synaptic terminal
respond to synaptic activity with Ca2+ waves
able to modulate synaptic activity by regulating EC ion levels and inducing post synaptic Ach receptor aggregation
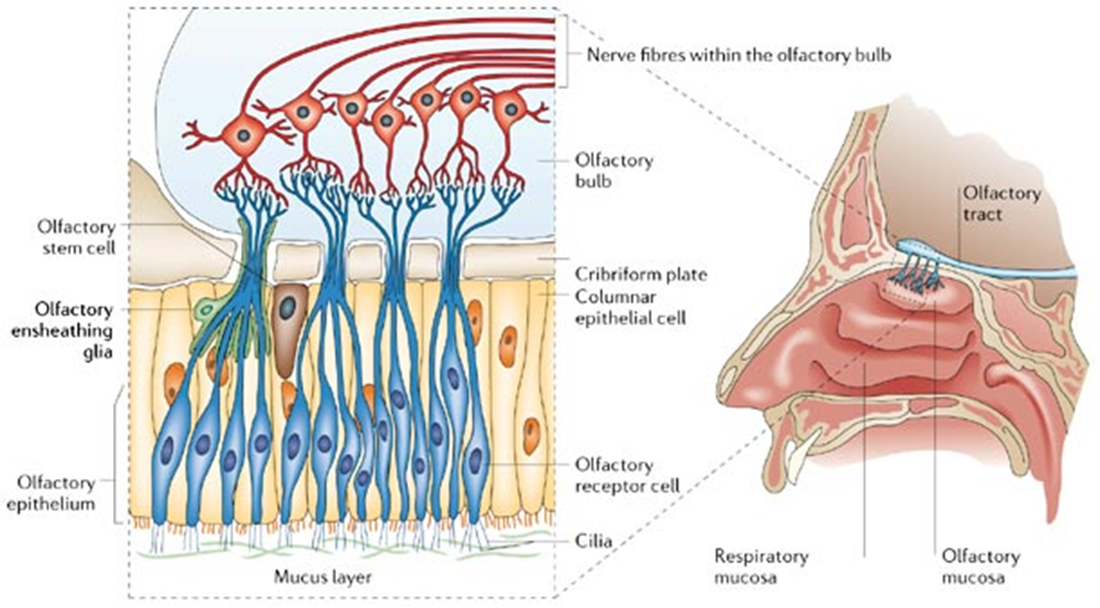
olfactory bulb ensheathing cells
myelinating cells that are not schwann cells or OG cells
found in olfactory bulbs
interesting type of glia - interface the PNS and CNS at the cribiform plate
myelinating cells moving towards olfactory plate
have roles similar to microglia —> phagocytose etc but also secrete neurotrophic factors which allow axons to cross glial scar
secrete glial markers similar to astrocytes (GFAP, s100, p75)) but also radial glial markers such as nestin and vimentin
due to their mixed role and allows cells to cross glial scar they have been proposed as a therapy to spinal injury
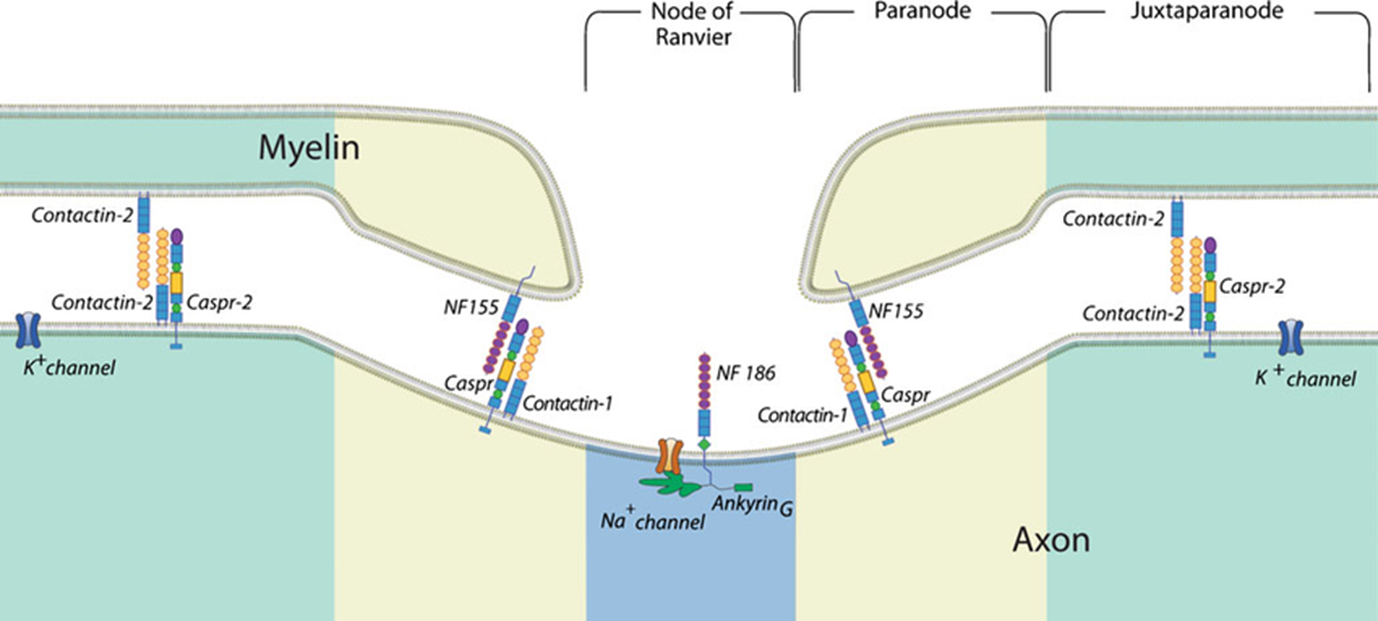
myelin
fatty insulated layer that facilitates saltatory conduction
myelin sheath wrapped arund axons to form concentric layers of lamella
every membrane has a different composition
myelin leaves gaps - nodes of Ranvier which are specialised axonal areas where action potentials can propogate
myelin sheath between the nodes of ranvier = inter nodes
specific molecules which allow for interaction of membrane and OGs
close to the node = paranode (closest to NOR) and juxtaparanode (closest to the internode)
moleular interactions at the paranode and juxtaparanode determine the clustering of K+ and Na+ channels that are key for saltatory conduction
E.g: contactin 2 and Caspr 2 are only expressed in juxtaparanode annd allow K+ channels to not invade node of ranvier
at the paranode: contactin 1 and Caspr which interacts with NF155 on the membrane of myelin cells
molecular patterning determines function of the compartments
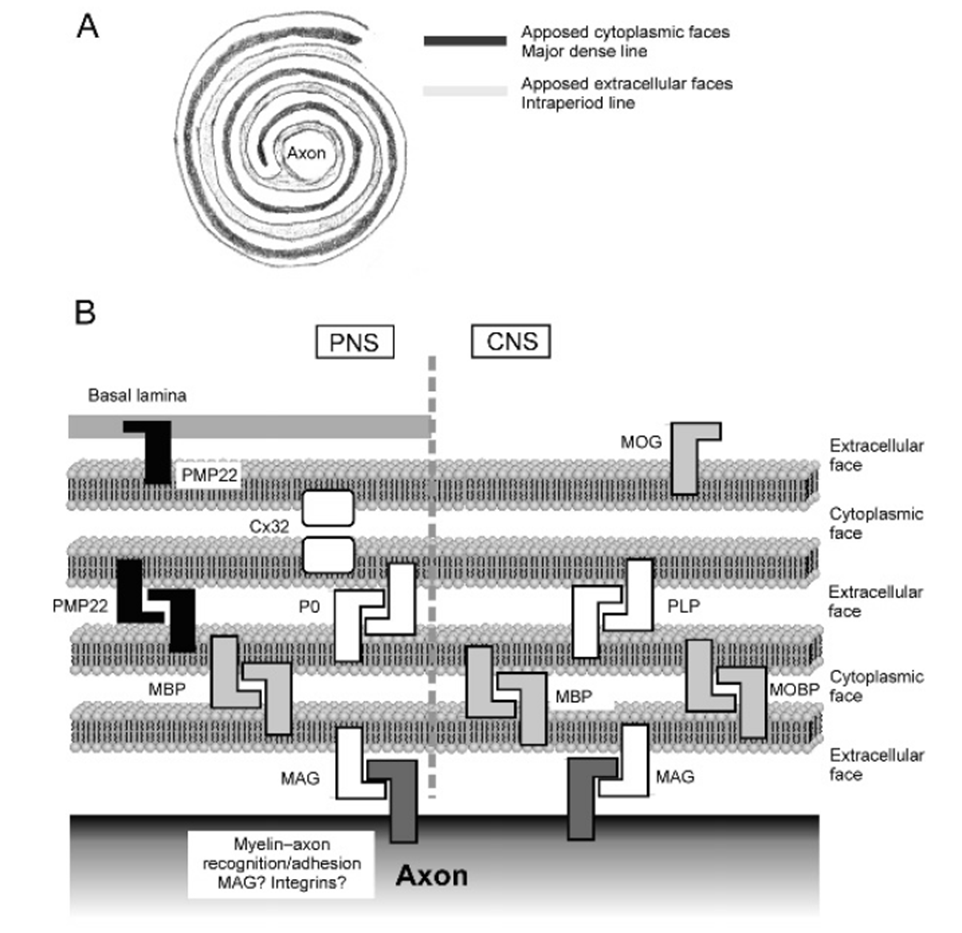
myelin: lipids
extracellular face and cytoplasmic face alternating
composed 70% of lipids, majority are cholesterol, some glycolipids, some phospholipids in a ratio (4:3:2)
its rich in glycosphingolipids, mainly GalC which is used as a marker
composed of gangliosides (complex lipids present in grey matter of the brain) which differs in the CNS from the PNS
in the PNS LM1 and GM3, in the CNS GM4
myelin: protein
the other 30% of myelin is composed of protein, mostly shared between CNS and PNS
in the CNS main ones are MBP, PLP which fuse EC and cytoplasmic faces (also present in PNS myelin but the functions unclear)
in PNS main protein is P0, mediating fusion of lamellae, but PMP22 and Cx32 are also important for keeping the membrane connected
MAG is present in both CNS and PNS —> important for connection of pile layers with axons, bind to specific gangliosides on the surface of the axon
myelination - process
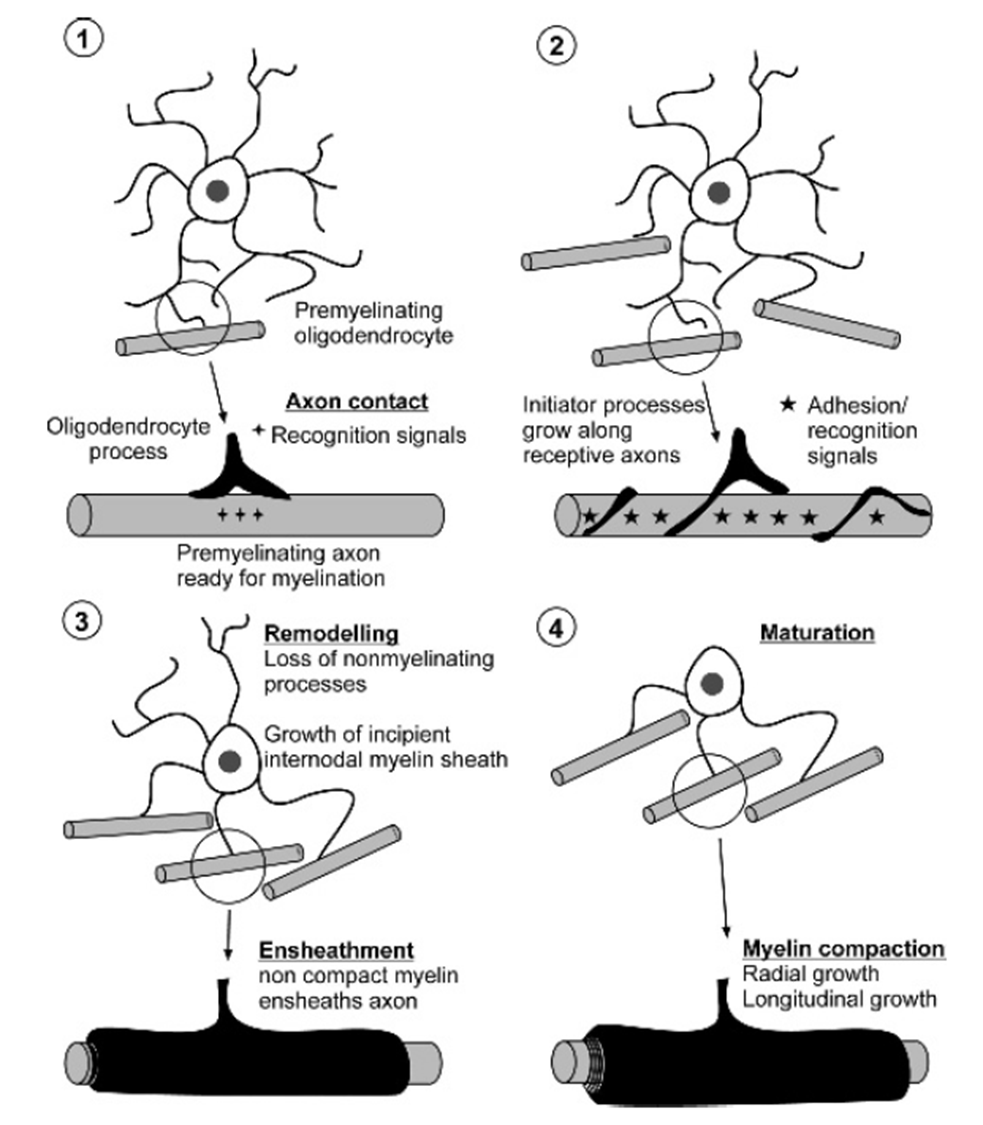
PHASE 1: Axonal contact
only if axon grows thicker than 0.7um in the PNS or 0.2um in the CNS
loss of NCAM from axonal surface triggers myelination, similarly L1 expressed at premyelination, tagging the axons to be myelinated
partner molecules in myelinating cells completely resolved
contact with axons triggers differentiation of OPCs into oligodendrocytes, starting to express myelin products (GalC, CNP, MBP etc)
PHASE 2: axon ensheathment and establishment of internode segment
extension of initiator process that spirals along the axon (using MAG and PLP to stick)
myelination of multiple axons follwed by remodelling phase when non ensheathment processes are lost
initial clustering of Na+ channels around nodes of Ranvier (happens in multiple axons at one time)
PHASE 3/4: remodelling and maturation
subsequentl wraps of myelin are produced which fuse to eachother dependent on PLP and MBP
maturation of nodes of Ranvier (synchronised expression of molecular pairs of axons and myelin)
some of the inital connections may be lost (remodelling)
when the final no of axons myelinated all other processes of OG lost
multiple sclerosis
immune system develops and autoimmune attack of the CNS forming plaques of lesions
generation of autoantibodies against myelin comonents
effects mostly the CNS and spinal chord
can allow Abs into the brain
most common type is relapse and remittance MS where the phases of demyelination are followed by remittance
commonly involves white matter
damages oligodendrocytes, causes demyelination
there are attempts to remyelinate but they are not complete
PATHOPHYSIOLOGY
key cause is BBB breakdown; causes entry of T cells
antibodies enter and recognise myelin
followed by chronic inflammation —. recruit immune cells of CNS (astrocytes, microglia)
contributes to the cycle of damage leading to inflammation
progression of MS
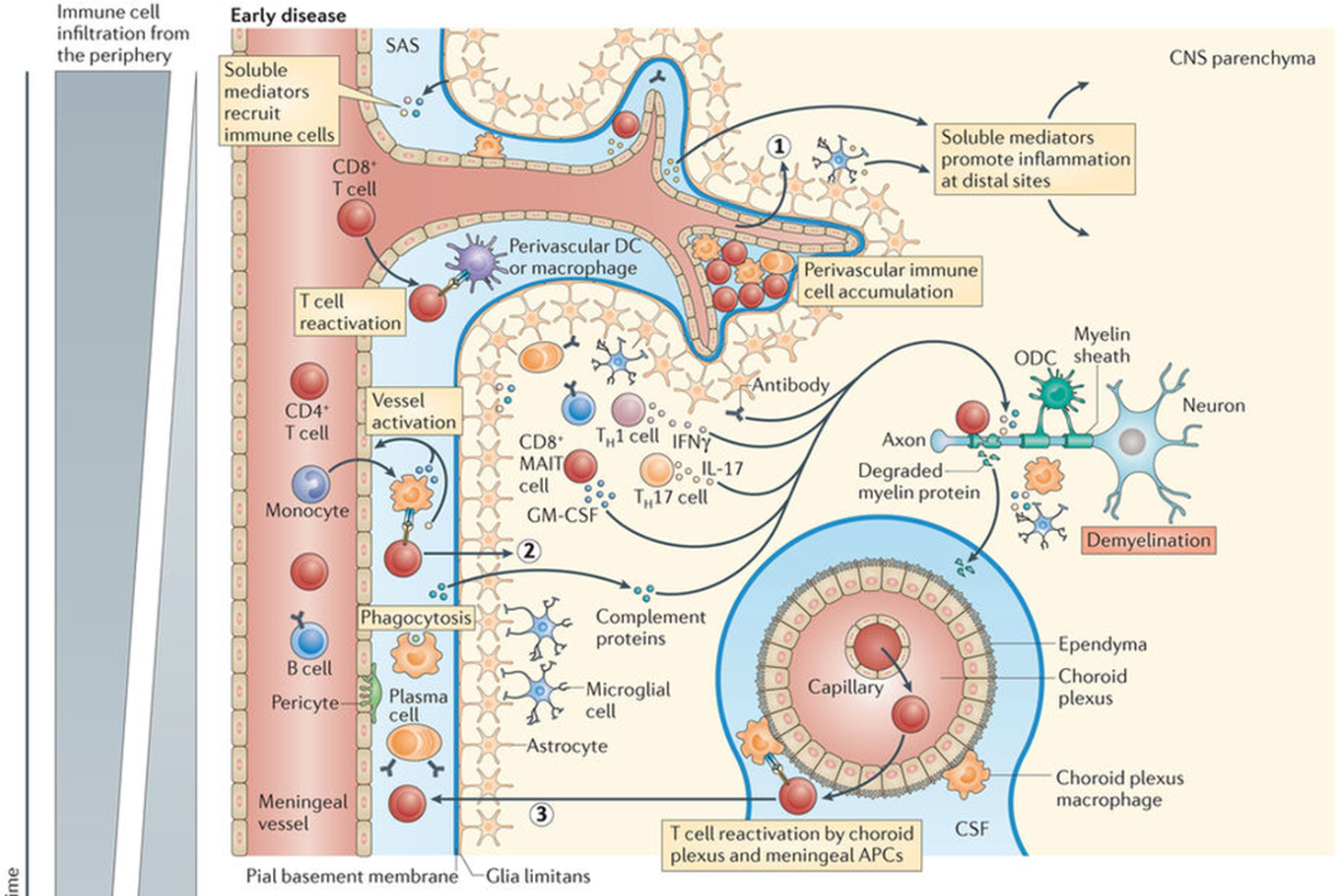
PHASE 1: early disease
antibodies are in circulation but brain is protected by BBB
can lead to immune cell accumulation in perivascular space (cytokine production which leads to leakiness of BBB)
antibodies can now attack white matter of the CNS
this brings in T cells which are able to permeate BBB and lead to destruction of myelin and damage to axon
once the myelin is degraded, reslts in degradation products in spinal fluid
when it reaches new ganglia it causes another cycle of reactivation
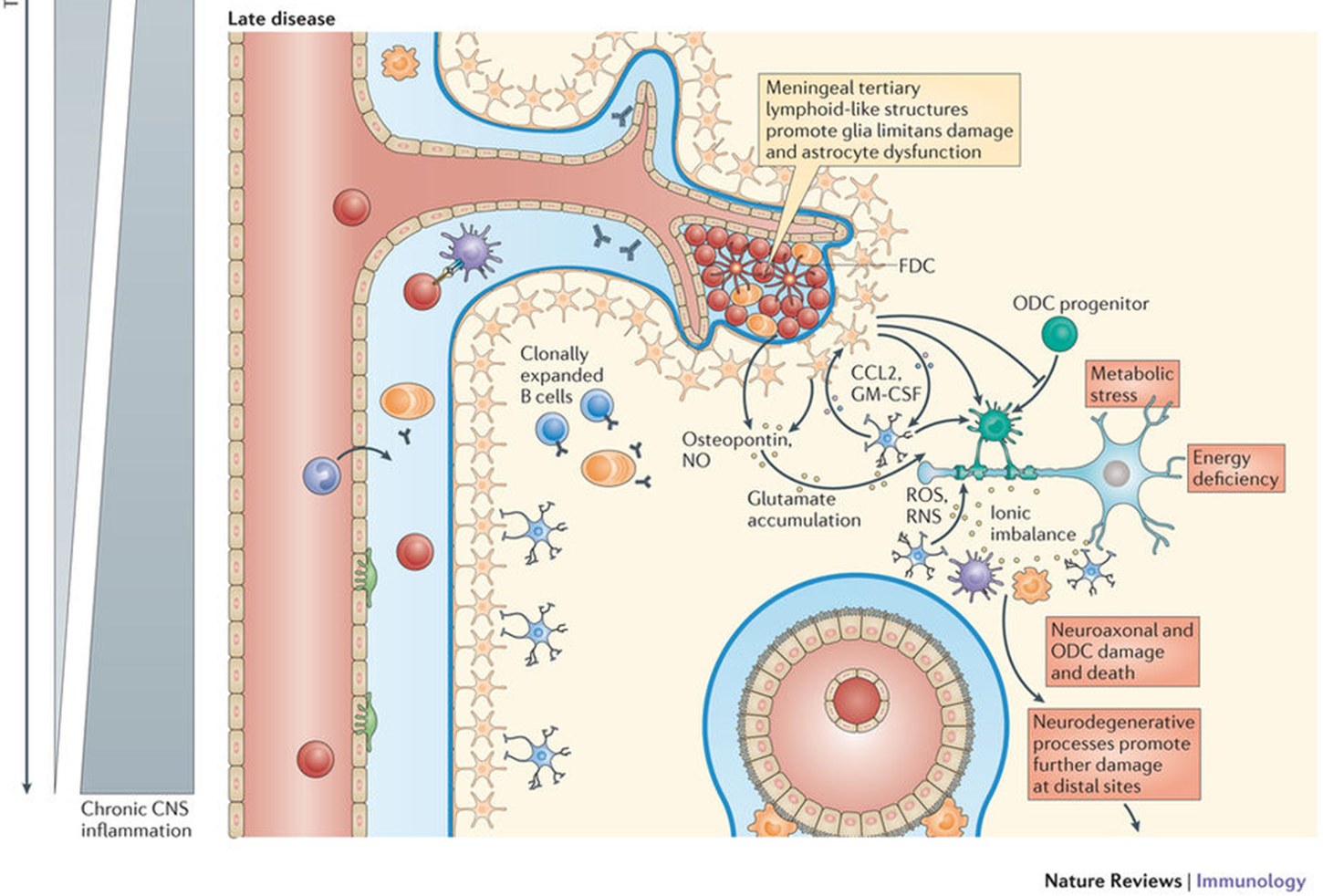
PHASE 2: chronic phase
immune cells and cytokines engage miroglia in cycle
E.g: chemokines, CCL2, GMCSF which activate reactive O species
activation of glia also leads to activation of oligodendrocyte precursor cells
damage to the myelin results in hanges such as Na+ channel rearrangement which affects neuron fuction
in long term the axon damage can result in astrocytes forming a glial scar
remyelination is often partially successful - able to tell via MRI (but will also never return to the original state of myelination)
microglial surveillance
mosaic like distribution with “territory” to protect
active protection
protects from
extravasion of unwanted molecules
cells that need to be removed
damage to the BBB
microglia extend their processes to limit and react to damage
much more rapidly than other cells
microglial diversity
show morphological diversity - observed via stain
structural axonal cues can dictate this
regional density differences:
hippocampus CA1 region is more dense, thalamus density is lower
grey matter/white matter density is different
have different turnover rates
slow in the cortex, fast in dentate gyrus
fast turnover means slower replacement potential
able to drive transcriptional differences in microglia samples by either cutting them post morten and sequencing or just sequencing
some core markers - these always present in microglia
also environmental dependent transcriptional molecules which can eb turned on/off depending on the environment
full maturation required environmental factors
gene expression profile in diseased microglia not neccessarily driven by microglia but by interaction with alzheimers brain
basic functions
ability of microglia to remove synaptic elements
ability to phagocytose
are microglia responsible for removal of synapses?
dorsolateral geniculate nucleus has territories for each eye (majority contralateral, some ipsilateral)
add stain to each side of teh eyes retinal ganglion cells and you can tell where the retinal ganglion cells project to in the dorsolateral geniculate nucleus
at some point we must get rid of some synapses to rewire circuitry so we retain some contrilateral projections
we find some of the tracer in microglia —> phagocytose some of the synapse
area which ends up being contralateral area the stain in microglia from ipsilateral fibres
remove synapses which must be removed
not known whether microglia are responsible - suggested that complement receptor 3 drives phagocytosis but microglia responsible for the degradation
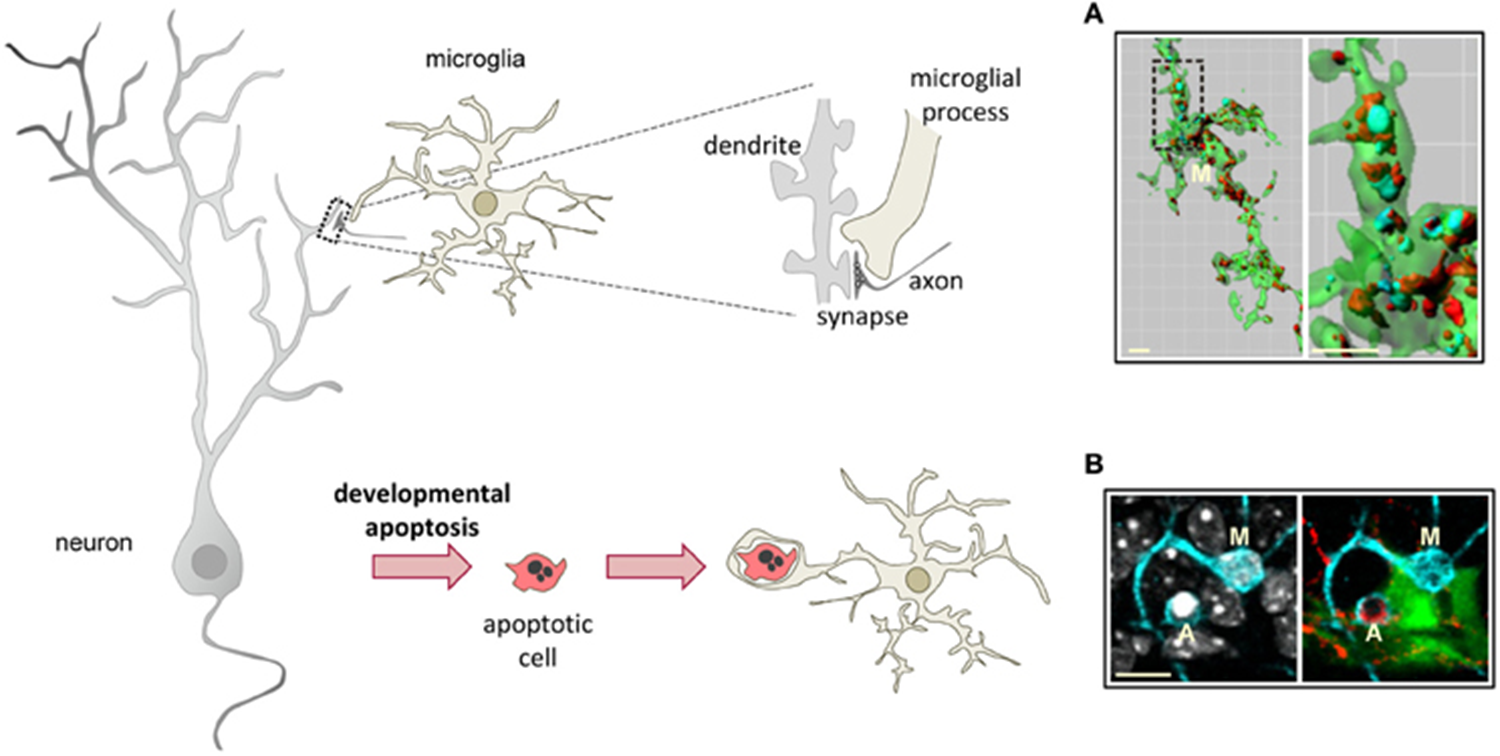
clearance of apoptytic cells
form phagocytic pouches, found to contain nuclei
metabolically demanding
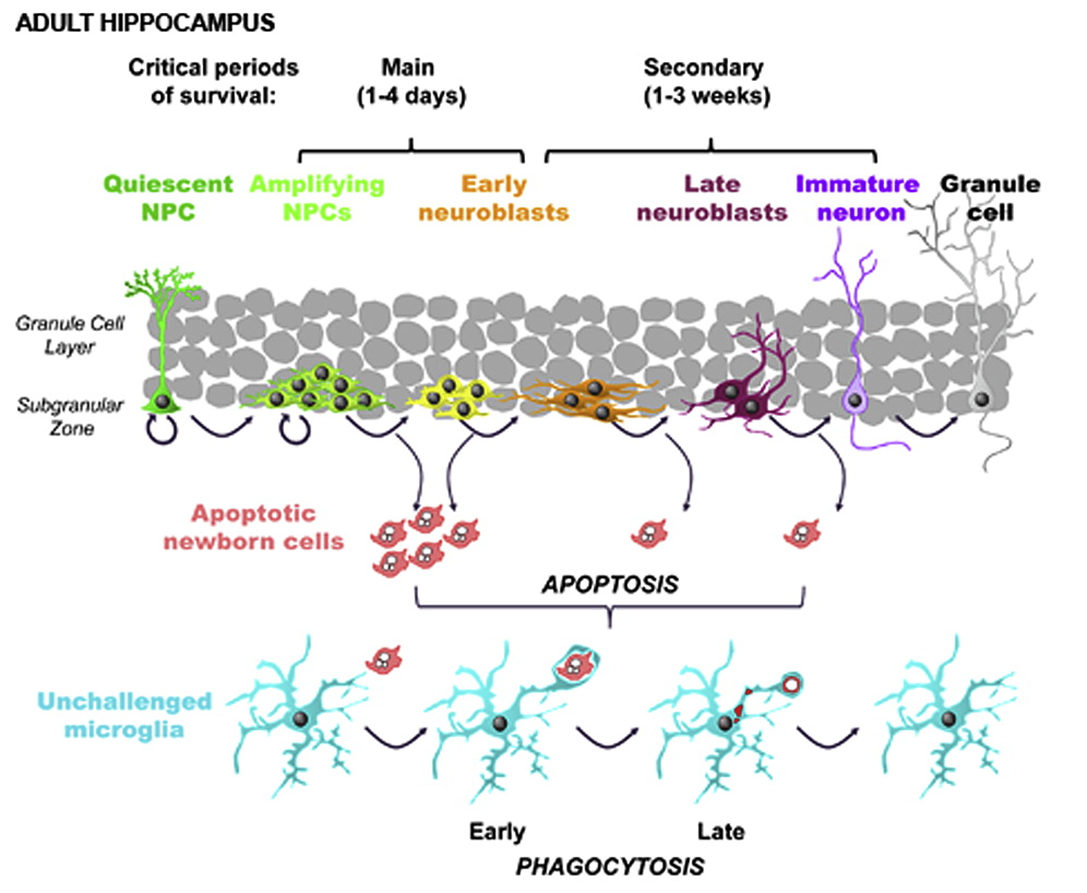
occurs in dentate gyrs - contains cells with neurogenic potential
huge no of radioglia produce amplifying neuroprogenitor cells (excess of cells)
these need to be removed by microglia
huge no of cells cleared
immune roles of microglia
equipped with molcules that allow them to react to environment
M1/M2 activation, M1=inflammation, M2= antiinflammatory response
no longer tak about M1/m2 response
population of microglia in steady state which can develop into a range of phenotypes which can change between eachother
gradient responses dependent on metabolic states, proteins etc (likely dictated by genetic background)
alzheimers
innate immunity is a key driver for alheimers
cognition deteriorates when infected
many genes in associated with alheimers are associated with immunity
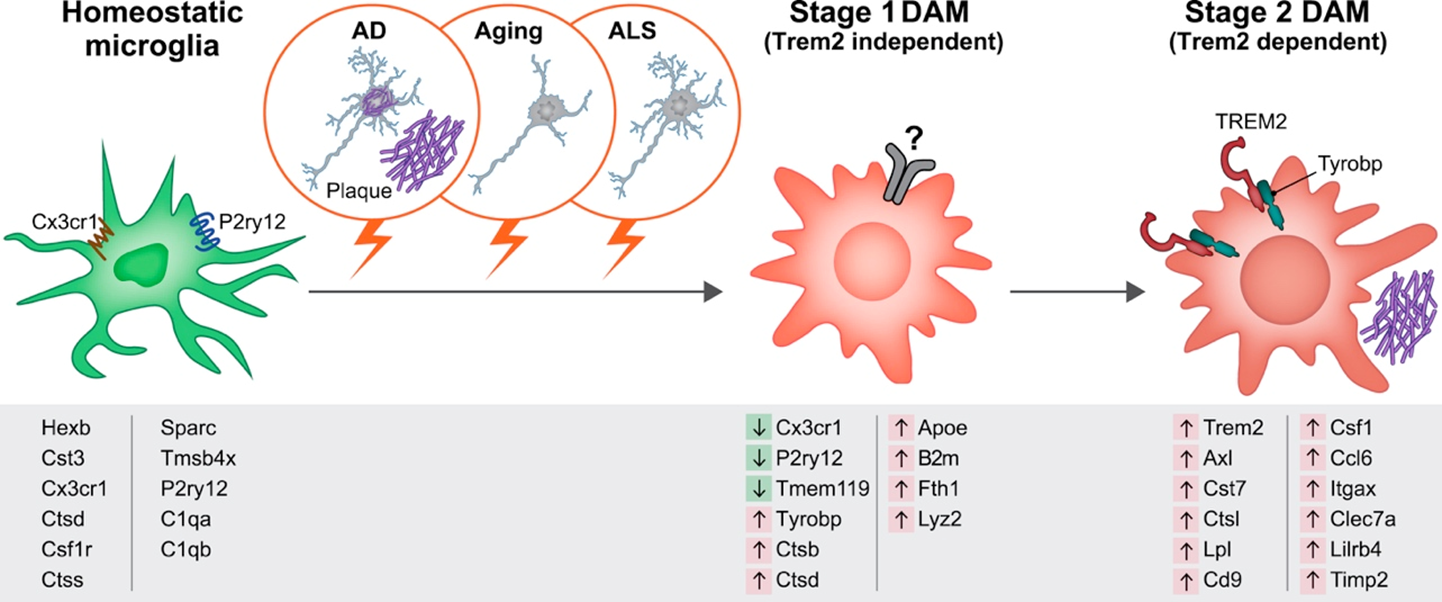
homeostatic microglia progress to dff phenotype in a 2 step process:
STEP 1: convert into disease associated miroglia
STEP 2: uses trem 2 to convert to diseased population of microglia which look nothing like normal microglia —> limits the growth of amyloid pathology
in disease, huge increase in microglia (not specific to alzheimers)
accumulation of amyloid beta, previously thought that this drove neurodegeneration
however, we can intervene and prevent degradation by altering microgia
microglia transducers of disease —> at some point they become involved ad amyloid beta drives the diseased phenotype