✅ Unit 2 - Exchange of materials
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
99 Terms
Properties of carbon
ability to form covalent bonds (strong)
ability to bond with same or different atoms and form chains of any length
ability to form single + double covalent bonds (single allow rotation)
macromolecule
A giant molecule created by atoms covalently bonded to one another (monomer + monomer)
4 main classes of macromolecules
carbohydrates
lipids
proteins
nucleic acids
types of monosaccharides
pentose
ribose
deoxyribose
fructose
hexose
galactose
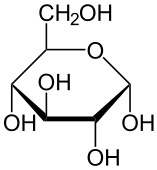
alpha glucose
subcategories of carbohydrates
monosaccharides
disaccharides
polysaccharides
subcategories of lipids
triglyceride
steroids
phospholipids
subcategories of proteins
polypeptides
structural
enzymes
transport
antibodies
animo acids
essential
nonessential
subcategories of nucleic acids
DNA
genes
chromosomes
RNA
mRNA
tRNA
rRNA
draw the ring form of alpha glucose
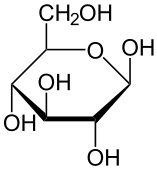
draw the ring form of beta glucose
properties of glucose
Soluble: due to multiple hydroxyl groups that form h-bonds with water = easily transportable in bloodstream
transportability: effectively transportable so it’s an energy carrier
chemical stability: relatively stable and doesn’t degrade easily over time (=good to store energy)
energy yield: oxidation of glucose during cellular resporation yields ATP
hydrolysis reactions
catabolic reactions where H20 molecules break covalent bonds between monomers from polymers
glycosidic bond
between monosaccharide units creating a disaccharide
______ and _____ are reversible
hydrolysis and condensation are reversible
condensation reactions
anabolic polymerisation reactions where 2 molecules join together where one molecule loses a hydroxyl (-OH) group and the other losing (-H) to create H2O as a byproduct
draw produce of alpha glucose + alpha glucose
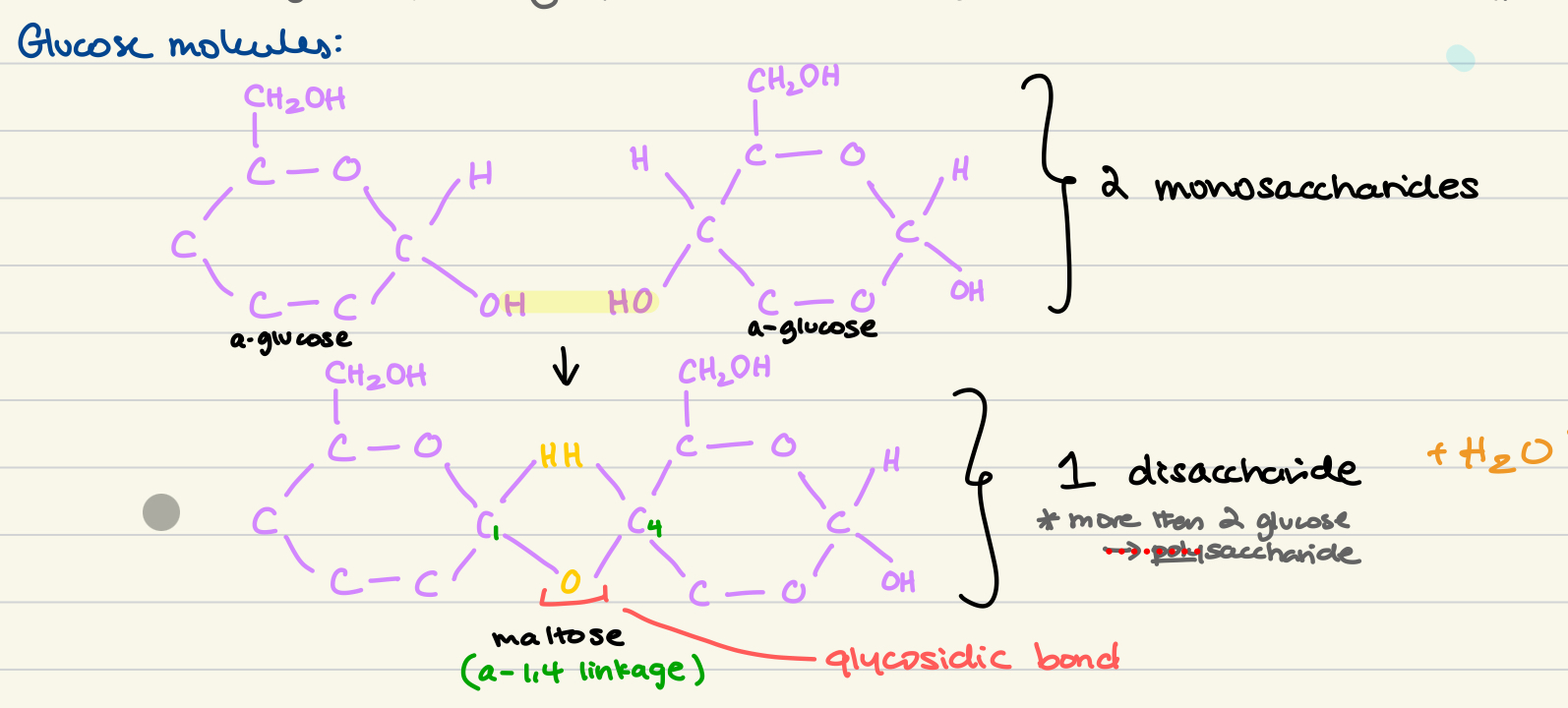
numbering carbons in a-glucose
carbon 1 has no attachments, adjacent to O
carbon 5 has a branch of c connected
alpha = OH on C-1 facing downwards
beta = OH on C-1 facing upwards
which polysaccharides are used as energy storage
glycogen
starch
amylase
amylopectin
glycogen as energy storage in animals
Highly branched (α-1,4 & more frequent α-1,6 than amylopectin) → very compact + rapid mobilization. Stored mainly in liver & muscles. Insoluble, doesn’t disturb osmotic balance.
starch as energy storage in plants
Made of amylose (α-1,4) and amylopectin (α-1,4 and α-1,6). Compact due to coiling (amylose) and branching (amylopectin). Insoluble, so doesn’t affect osmosis. Glucose units easily added/removed for energy use.
Amylose vs. Amylopectin
Amylose: Unbranched, α-1,4 bonds → coils into helix → compact but slower to hydrolyze.
Amylopectin: Branched (α-1,4 + α-1,6) → more ends → faster glucose release.
Both make starch efficient for storage in plants.
cellulose + function as a structural polysaccharide in plants
made up of beta-glucose monomers linked by 1,4-glycosidic bonds.
alternating orientation of beta-glucose = straight chains.
bundled together in microfibrils, + cross-linked with h-bonds
prevent the cellulose from bursting and help regulate osmotic pressure
High tensile strength is due to covalent bonds within the microfibrils
glycoproteins are a _____ of a _______ and a ______ , fundamental in cell ______ acting as ______ as well as for cell ______
glycoproteins are a combination of a carbohydrate and a protein, fundamental in cell recognition acting as receptors (identify + destroy foreign cells) as well as for cell adhesion
structure of glycoprotein
conjugated proteins with carbohydrate as the prosthetic group, embedded in the plasma membrane projecting out into the exterior environment
How do ABO antigens affect blood transfusions?
Red blood cells have glycoproteins with different oligosaccharides:
Type A and B have unique 5th monosaccharides
→ AB can't donate universally
Type O lacks this 5th sugar = base structure shared by all
→ universal donor (no “foreign” sugar triggers immune response)
examples of carbohydrates
glucose
sucrose (plants)
cellulose
glycoproteins
starch
glycogen
glycolipids
molecules with carbohydrates linked to lipids
glycolipids’ role in cell recognition
helps immune systems distinguish between self vs. non-self cells to destroy pathogens
lipids
organic molecules that insoluble in water and are found in the structure of cell membranes + used as energy stores
common examples of lipids
oil
fats
waxes
steroids
phospholipids
roles of lipids
long-term energy storage/chemical energy
structural integrity (phospholipid bilayer)
communication (hormones to start/stop protein production)
thermal insulation
lipids as energy storage
Triglycerides = long-term storage as more dense
Carbohydrates = short term
ATP = immediate
properties of triglycerides
chemically stable: energy isn’t lost over time
immiscible: naturally forms droplets in cytoplasm and doesn’t affect osmotic balance
energy dense: 2x j/g than carbs and is useful for animals that move (more energy, less space)
liquid at body temp: acts as a shock absorber
poor heat conductors: thermal insulators for animals to conserve heat
triglycerides as energy storage in adipose tissue
specialised group of cells (adipose tissue) is located beneath the skin and around some organs (kidneys)
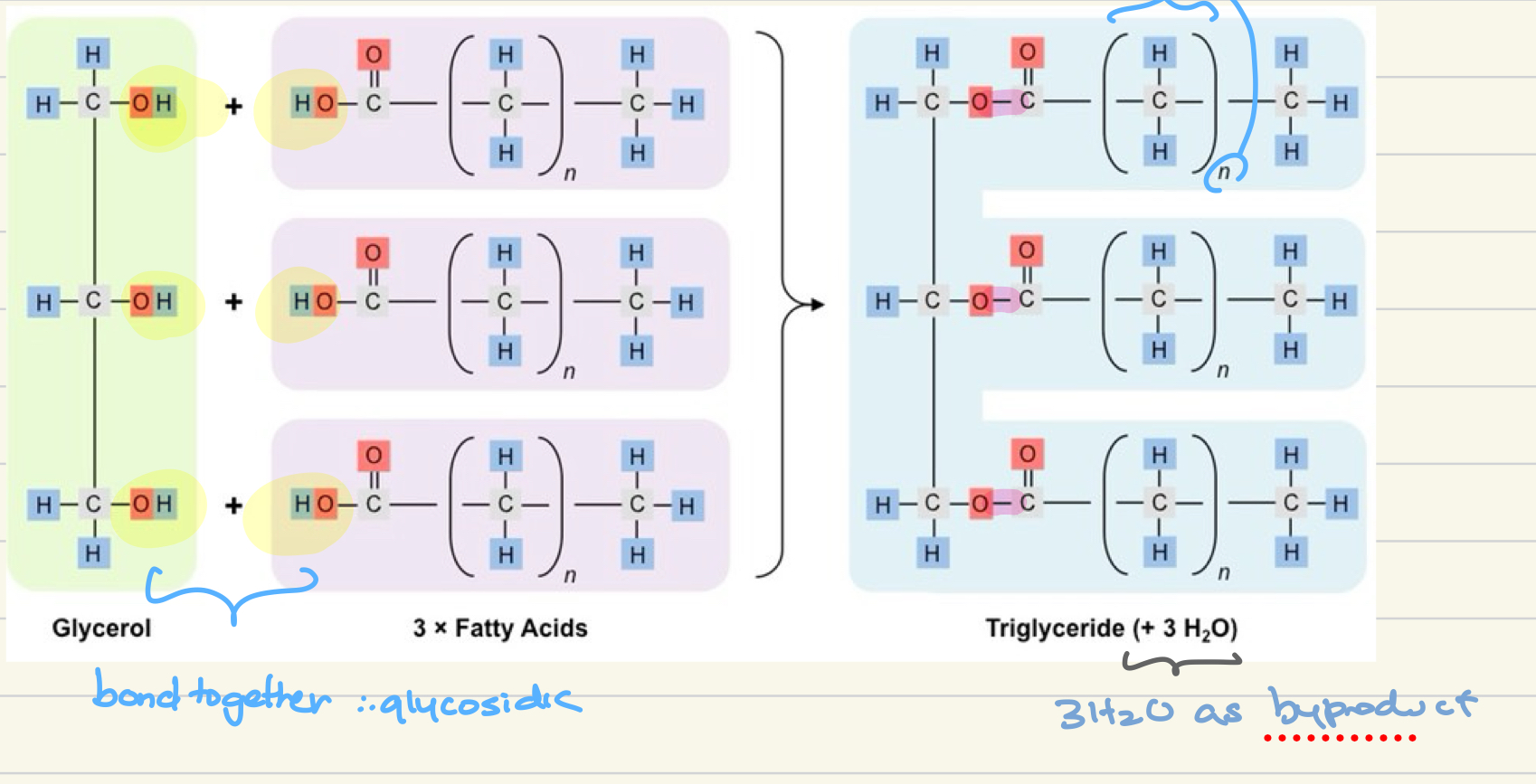
How are triglycerides formed?
through condensation reaction with h2o as byrpoduct where glycerol and 3 fatty acids form glycosidic bonds to become a triglyceride
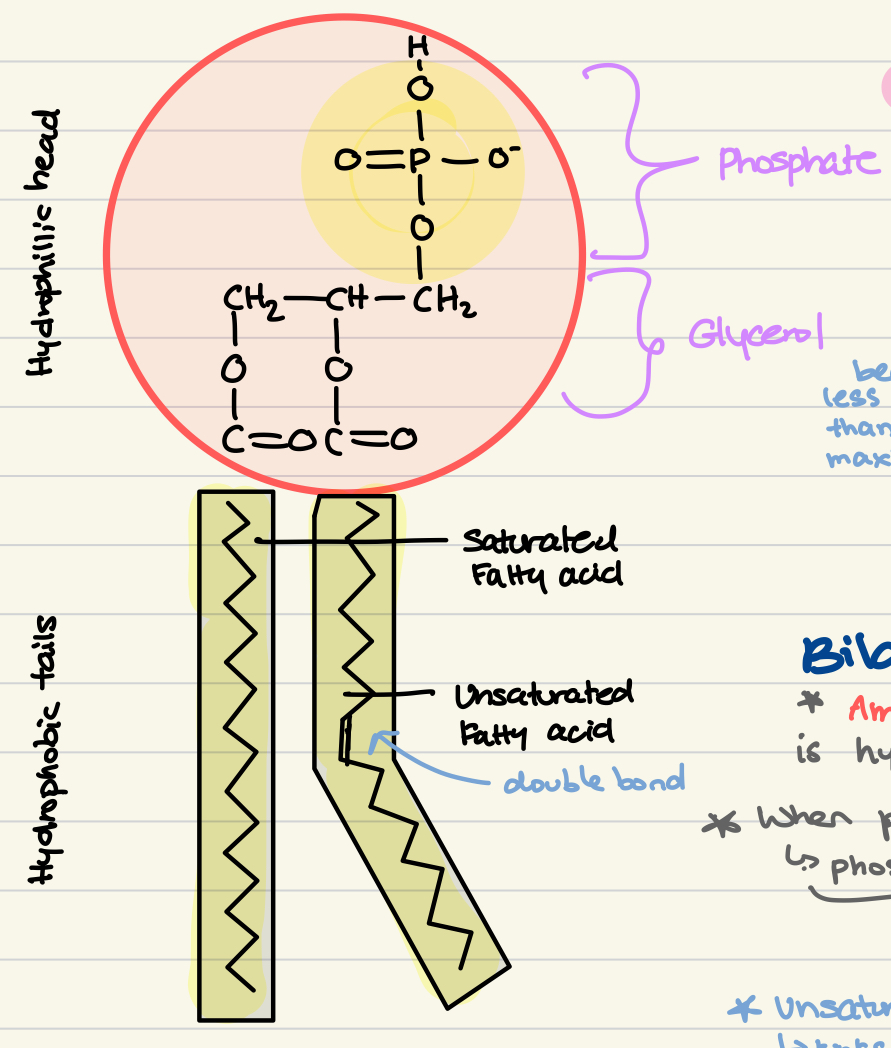
Phospholipid structure
Condensation reaction with ester bonds
Hydrophillic Head = 1 glycerol + 1 phosphate group
Hydrophobic Tails = 2 fatty acids
Saturated → single bonds (straight)
Unsaturated → double bond
trans-fat: straight chain
cis-fat: kinks (lower MP due to easy separation)
unsaturated fatty (__-fat) are _____ to stack on top of each other due to their ____ and thus reduce ___ in blood vessels, decreasing chances of ____
unsaturated fatty (cis-fat) are harder to stack on top of each other due to their kinks and thus reduce clogs in blood vessels, decreasing chances of stroke
amphipathic molecules
part of the molecule is hydrophillic, the other part is hydrophobic
The ______ phosphate heads are _____ to the water and face ______, while the _______ tails are packed together ____ from the water, creating a _____ layer
The hydrophilic (water-loving) phosphate heads are attracted to the water and face outward, while the hydrophobic (water-fearing) tails are packed together away from the water, creating a double layer
non-polar steroids
4 rings of carbon atoms (17 C total)
1 cyclohexane
1 cyclopentane
Low carbon to oxygen proportion (lipid)
mostly hydrocarbon and thus hydrophobic
can pass through bilayers
ex. oestradiol, testosterone
general structure of an amino acid (AA)
amine group (basic) bonded to the central carbon bonded to the r’ group and carboxyl group (acidic)
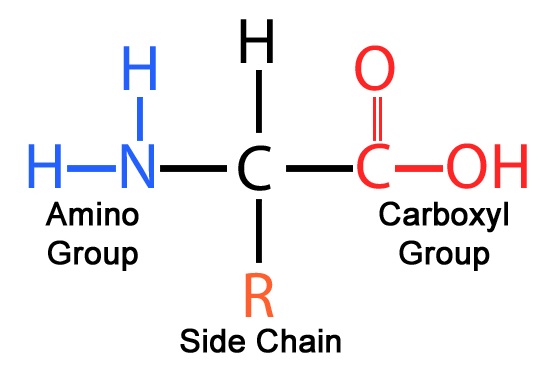
r groups in amino acids
differs from one AA to the next
determines the properties of the AA
polarity (acidic or basic)
h-h bonds
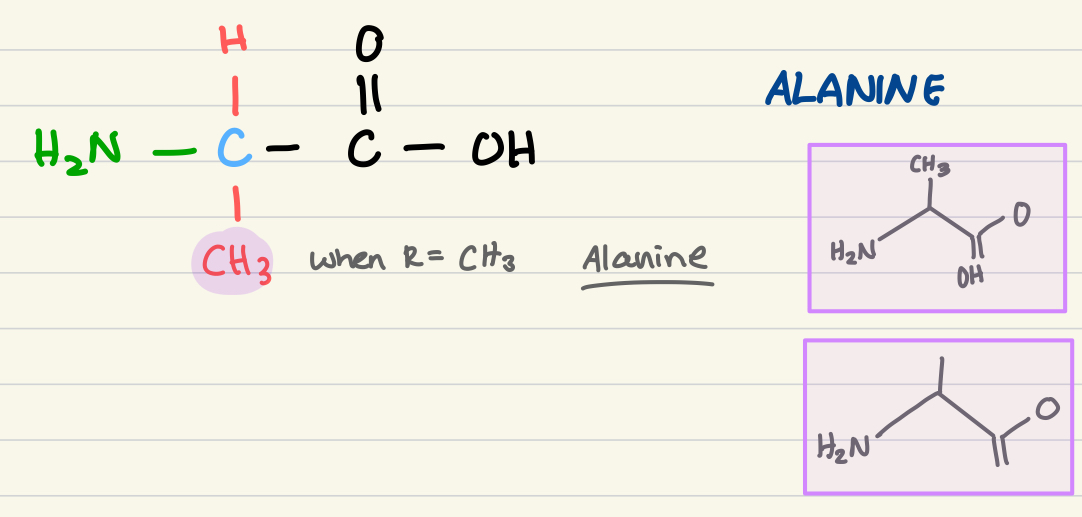
structure of alanine
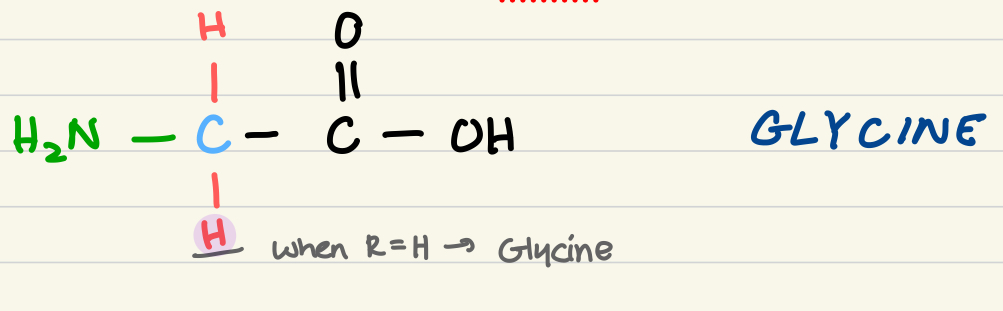
structure of glycine
amino acids are bonded together by _____ bonds through _____ reactions (H2O ______)
amino acids are bonded together by peptide bonds through condensation reactions (H2O byproduct)
polypeptides
A chain of amino acids that is linked together by peptide bonds
disulfide bridges
covalent bonds that form between pairs of cysteine amino acid residues → S-S bond which affects the function of the AA)
____ containing R-groups are ___-_____
sulfur containing R-groups are non-polar
adding ___ group increases _____ charge
adding amine group increases positive charge
adding ___ group increases _____ charge
adding carboxyl group increases negative charge
formula to calculate # of bonds in polypeptide
20n, n = amino acid #
Denaturation definition
the process where a protein loses its 3D structure due to the breaking of non-covalent bonds like hydrogen bonds and hydrophobic interactions
Primary protein structure
polypeptide chain with no definite 3D structure, can de depicted with letters
Secondary protein structure
polypeptide chain folding and coiling into regions of alpha helices and beta-pleated cheets due to H bonds (C from carbonyl and H from peptide bond)
Tertiary protein structure
1 Polypeptide chain folded into 3D structure stabilised by R-group interactions;
Ionic bonds
Hydrogen bonds
(Covalent) Disulfide bridges: between pairs of cysteines
Hydrophobic interactions
Ionic bonds in tertiary protein structure
Occur when + and - R groups interact where the amine takes a P ion from carboxyl to become more + while making the carboxyl more -
Differentiate between Fibrous vs. Globular Proteins
Feature | Fibrous Proteins | Globular Proteins |
|---|---|---|
Shape | Elongated polypepties (long, strandish) | Folded polypeptides stabilised by R group bonds (round, spherical) |
Solubility | Insoluble in water | Soluble in water |
Function | Structural/support (ex. strength, rigidity) | Functional/metabolic/catalytic (ex. enzymes, hormones) |
Examples | Collagen, Keratin, Fibrin | Hemoglobin, Insulin, Enzymes |
R-Groups | Hydrophobis AA in center to stabilise tertiary structure | Hydrophillic AA on surface to allow solubility |
Stability | More stable | Less stable |
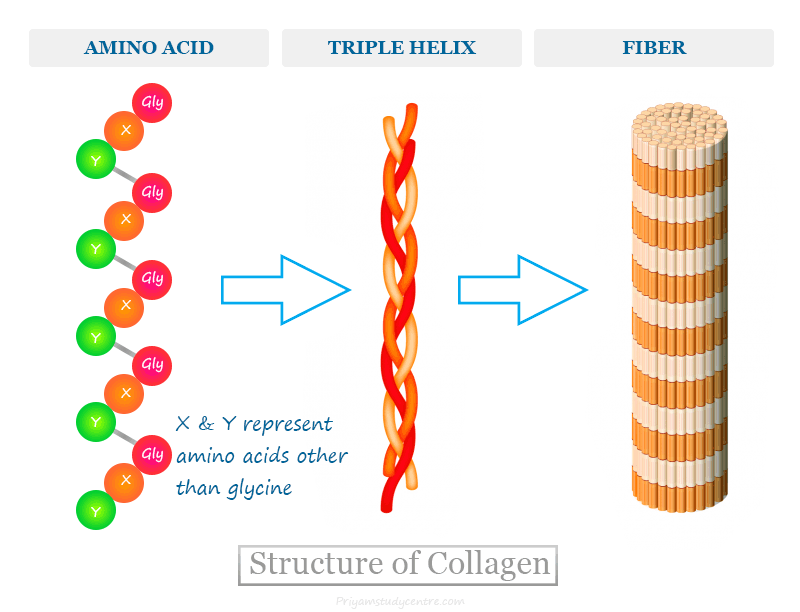
Collagen as a fibrous protein
Made of 3 polypeptides wound into a triple helix (P-G-X repeating sequence)
Rope-like structure → high tensile strength
R-groups face outward → allows functional variation
Found in tendons, ligaments, skin, and bones
Insulin as a globular protein
Small, compact globular hormone
Soluble in blood → easy transport to target cells
Binds to insulin receptors (specific conformation only bindable to its receptor) → regulates blood glucose
Functional role in metabolism, not structural
Role of R-groups in Integral proteins
Hydrophobic AA R-groups face outward towards membrane’s non-polar core = anchors protein
Hydrophilic R-groups face cytoplasm = interaction with water or solutes
Role of R-groups in Transmembrane proteins
Hydrophobic R-groups face out in membrane-spanning regions = interact with phospholipid tails
Hydrophilic R-groups face inward (channel) or on exterior ends = interact with aqueous environments + stability + function in transport/signaling
Role of R-groups in Channel proteins
Tunnel lined with hydrophilic AA = allows hydrophilic solutes to diffuse across hydrophobic core
Quaternary protein structure
2 or more polypeptide chains combined, can be conjugated or non-conjugated
Conjugated proteins
contain prosthetic groups, ex. haemoglobin with 4 polypeptides and haem groups that increases chemical and functional diversity
haem binds to oxygen allowing its transport
Non-conjugated proteins
purely made of AA with same linkage as tertiary structure
ex. insulin with 2 polypeptides linked by disulfide bridges or collagen with 3 polypeptides wound together
Simple diffusion
PASSIVE movement down a concentration gradient across the phospholipid bilayer for;
Small nonpolar/hydrophobic molecules (e.g., O₂, CO₂)
Small uncharged polar molecules (e.g., H₂O, ethanol) — limited permeability
Dynamic equilibrium
No net change in concentration with particles continuously moving to maintain equilibrium
Semi-permeable membrane allows certain ____ solutes and is freely permeable to _____ (ex. artificial membrane used in kidney dialysis)
Semi-permeable membrane allows certain small solutes and is freely permeable to solvent (ex. artificial membrane used in kidney dialysis)
Selectively permeable membrane allows passage of ____ particles through _____ diffusion and _____ transport (ex. chloride channel)
Selectively permeable membrane allows passage of specific particles through facilitated diffusion and active transport (ex. chloride channel)
Types of membrane proteins
Integral Proteins
Channel proteins
Transmembrane
Pump proteins
Peripheral Proteins
characteristics of channel proteins
diameter of pore is tailored so only one type of particle passes
bidirectional but normally higher to lower conc
facilitated diffusion
characteristics of pump proteins
use energy to carry out active transport
one directional
against the concentration gradient
interconvertible: atp switches between more → less stable configurations
characteristics of peripheral proteins
hydrophilic on the non-embedded surface
attached to the surface of integral proteins
1 hydrocarbon chain is inserted into membrane = anchor
osmosis
net movement of particles moving in and out of a cell due to differences in concentrations
aquaporins
integral water channels increasing membrane permeability to water
(ex. kidney cells to reabsorb water, root hair cells to absorb water from soil)
high membrane fluidity
lower density of phospholipids making the membrane more permeable, mix of saturated and unsaturated fatty acids.
low membrane fluidity
high density of phospholipid decreasing permeability, freezes easily
cholesterol’s role in membrane fluidity
High temperatures: holds together the phospholipids to stabilise and minimise permeability
Low temperatures: acts as a buffer to prevent solidifying
types/examples of endocytosis
pinocytosis (drinking)
phagocytosis (eating) (ex. macrophages engulf large particles, including bacteria, for digestion)
receptor-mediated endocytosis
example of exocytosis
cells release hormones like insulin (pancreatic beta cells) and other signaling molecules into the bloodstream
3 types of channels
voltage-gated channels
ligand-gated channels
mechanically-gated channels
example of a voltage gated channel
Sodium potassium pump (direct active transport)
Outline the processes of the sodium potassium pump / Describe an example of direct active transport
3 Na+ and 1 ATP bind to the pump
ATP dephosporylates becoming ADP and induces a conformational change opening the pump side facing the cytoplasm
3 Na+ ions are released outside the membrane and 2 K+ ions bind to the pump
K+ is released inside the membrane with releasing the Phosphate
Against the concentration gradient, inside is relative more - charged than outside (3+ go out, 2+ come in) creating an electrochemical gradient that creates an action potential
Describe an example of indirect active transport
Sodium-dependent glucose co-transporters
Indirect as ATP isn’t directly involved but it was in previous processes (sodium potassium pump)
Glucose ‘grabs onto’ the excess Na+ from the pump and goes back into the cell against it’s concentration gradient allowing glucose reabsorption (ex. small intestine)
Describe an example of a ligand-gated channel
Nicotinic acetylcholine receptor
Acetylcholine (exitatory neurotransmitter) binds to the receptor present at skeletal neuromuscular joints
Induces a conformational change opening the ion channel
Na+ ions diffuse down the concentration gradient
Inner cell becomes more positive and creates action potential through depolarisation
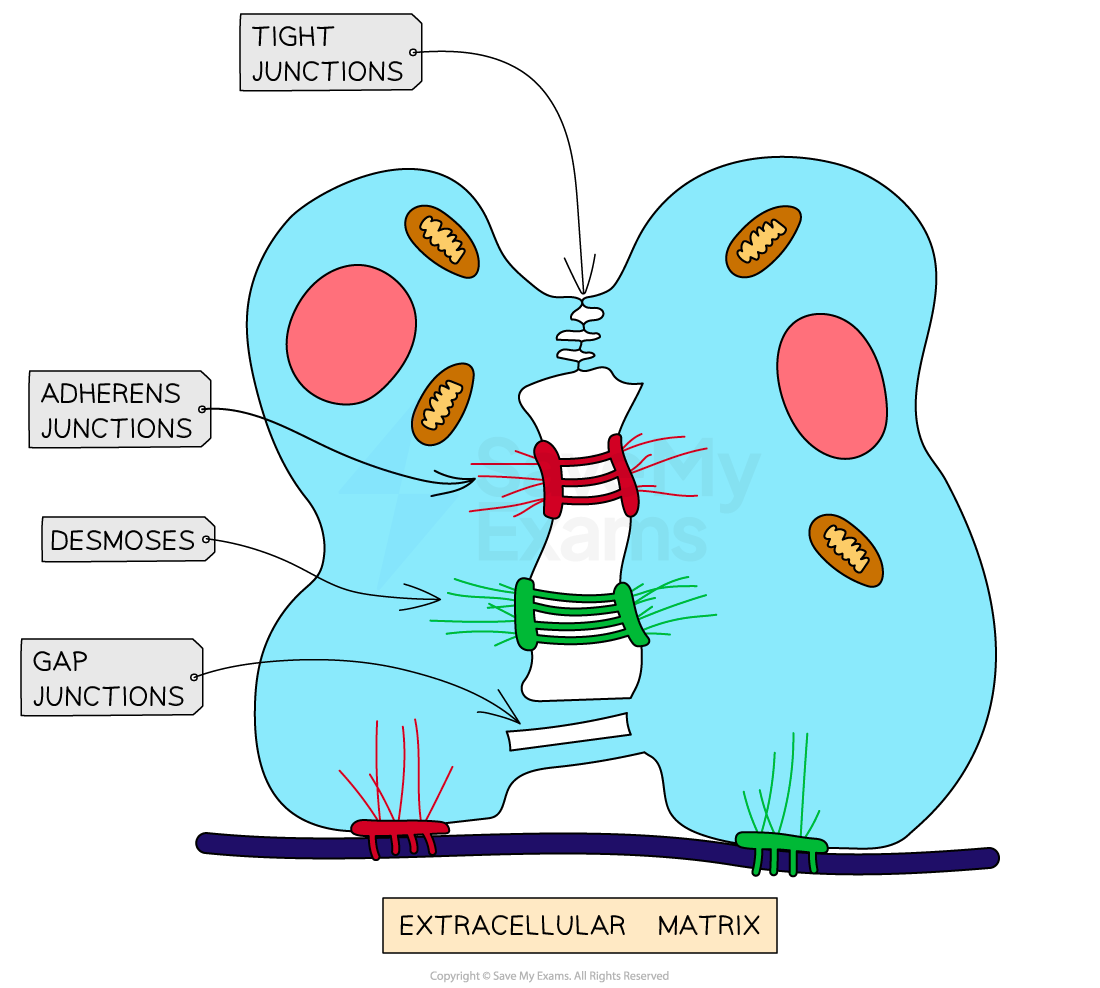
Cell Adhesion Molecules
type of cell surface protein that bind cells with other cells or with the extracellular matrix (containing supporting structures like collagen proteins)
in tumors they prevent cells from seperating and mitigate metastasis
Types of intracellular junctions (CAMs)
Tight junctions
Adherens junctions
Desmosomes
Gap junctions
Water potential
a measure (kPa) of the (potential energy of water / per unit of volume water).
highest is 0 so normally - values, (ex. -200kPa cell inside a -300kPa solution, water moves from inside the cell to outside)
formula for total water potential
total water potential = solute potential + pressure potential
Hypertonic effect on animal cells
Shrivelled / Crenated
Hypertonic effect on plant cells
Plasmolysed
Isotonic effect on animal cells
Normal
Isotonic effect on plant cells
Flaccid
Hypotonic effect on animal cells
Lysed
Isotonic effect on plant cells
Turgid
Hypertonic
A solution with a higher solute concentration than the cell, causing water to move out of the cell
Hypotonic
A solution with a lower solute concentration than the cell, causing water to move into the cell
Isotonic
A solution with the same solute concentration as the cell, resulting in no net movement of water