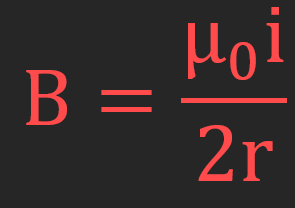Atoms and Quanta Equations
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
Intensity of black body radiation
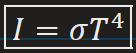
Power Radiated by a black body
Intensity times Surface Area
wavelength at which emitted intensity of a black body is maximum
Wein’s Constant/ Temperature
Einstein’s stopping potential
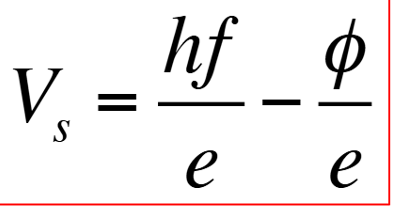
linear momentum of an incoming photon
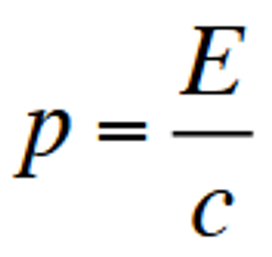
De broglie wavelength
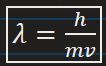
Heisenberg uncertainty principle for momentum
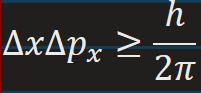
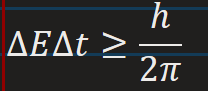
Heisenberg uncertainty principle for Energy
Kinetic energy of an electron in terms of potential difference
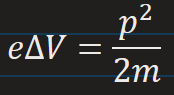
Energy Levels according to Schrodinger’s bound equation
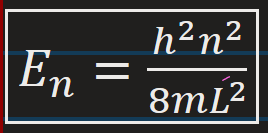
Bohr radius
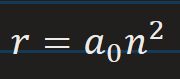
Bohrs claim about angular momentum of the orbiting electron
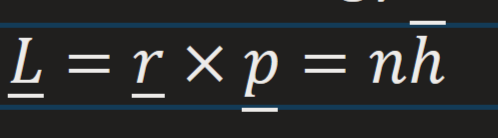
Schrodinger equation energy for an orbiting electron
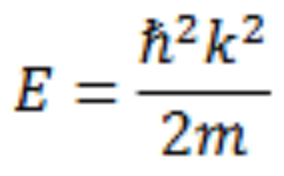
magnitude angular momentum of orbiting electron
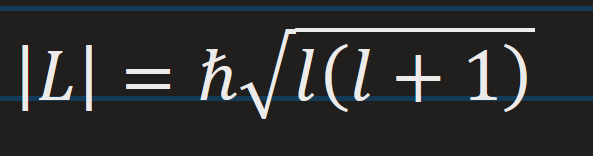
z component of the angular momentum of an orbiting electron
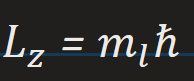
Spin-orbit coupling
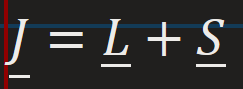
energy of Kalpha transition in an atom

Time Dependent Schrodinger
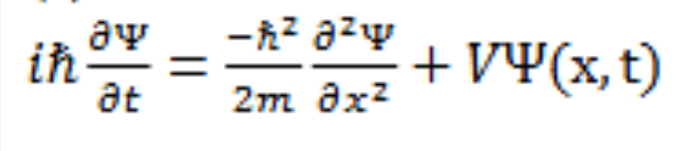
Time independent Schrodinger

Number of n2 in Rydberg Formula for the Lyman series and Balmer Series
1 and 2
Hydrogen Energy levels according to Bohr
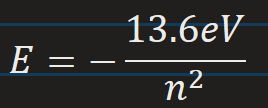
relativistic energy formula
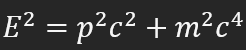
Magnetic moment of spin of an electron
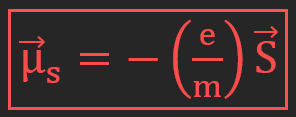
Interaction energy in a magnetic field

Bohr magneton formula
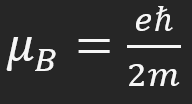
Magnetic field at the centre of a current-carrying loop