2. Period 3 elements reacting with oxygen
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What do period 3 elements form when they react with oxygen?
Oxides
Period 3 elements are usually oxidised to their… oxidation states.
highest
What is the exception to period 3 elements being oxidised to their highest oxidation states. Give it’s example.
Sulfur, it forms SO2 with a +4 oxidation state. The highest oxidation state for sulfur is +6.
What is needed for sulfur to be oxidised to its highest oxidation state? What is the formula?
high temperature
catalyst
SO3 with an oxidation state of +6
The more reactive metals (Na, Mg) and the non-metals (P,S) react … in air while Al and Si react more slowly.
readily
General formula of the formula for the making of all period 3 oxides.
element + oxygen → oxide
word and symbol equation for:
Na and O2
Reaction in air:
Flame colour:
sodium + oxygen → sodium oxide
Reaction in air: Vigorous
Flame colour: Yellow
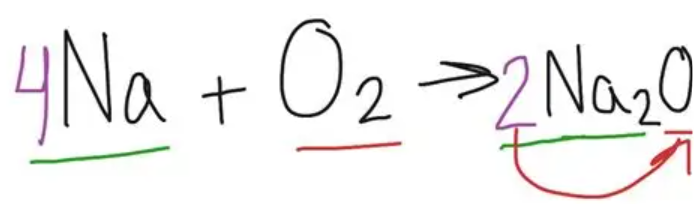
word and symbol equation for:
Mg, O2
Reaction in air:
Flame colour:
magnesium + oxygen → magnesium oxide
Reaction in air: Vigorous
Flame colour: Brilliant white
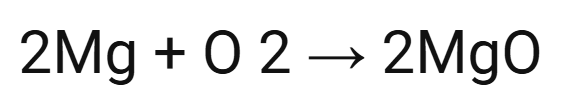
word and symbol equation for:
Al, O2
Reaction in air:
Flame colour:
aluminium + oxygen → aluminium oxide
Reaction in air: Slow
Flame colour: N/A
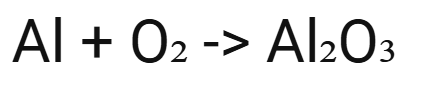
word and symbol equation for:
Si, O2
Reaction in air:
Flame colour:
silicon(s) + oxygen(g) → silicon dioxide(s)
Reaction in air: Slow
Flame colour: N/A
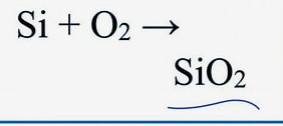
word and symbol equation for:
P, O2
Reaction in air:
Flame colour:
phosphorus + oxygen → phosphorus(V) oxide
P4(s) + 5O2(g) → P4O10(s)
Reaction in air: Spontaneously combusts
Flame colour: Brilliant white
word and symbol equation for:
S, O2
Reaction in air:
Flame colour:
sulfur + oxygen → sulfur dioxide
S(s) + O2 (g) → SO2 (g)
Reaction in air: Burns steadily
Flame colour: Blue