Properties of hydrocarbons final
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Water
Non toxic
Methanol
Highly flammable liquid
Toxic if inhaled, swallowed or in contact with the skin
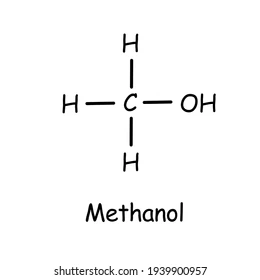
n-Butanol
Flammable
Poisonous gases are produced in fire
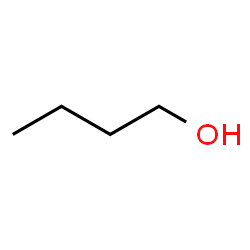
Ethylene glycol
Irritant
Aspiration hazard if swallowed
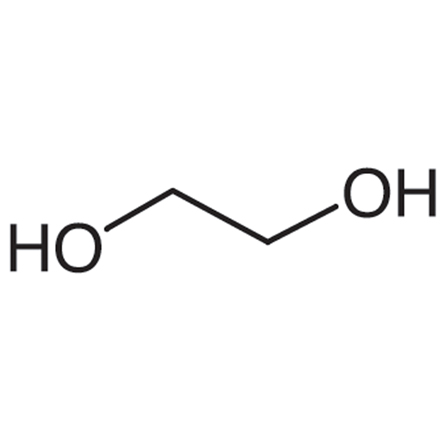
Acetone
Can cause eye irritation
If swallowed dizziness and drowsiness
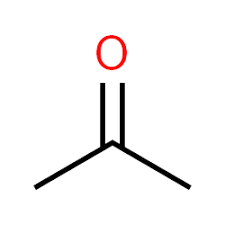
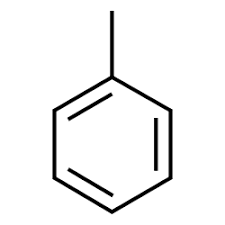
Toluene
Eye and nose irritation, tiredness and confusion
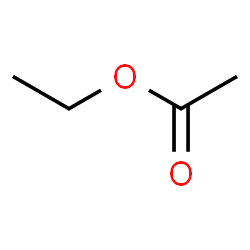
Ethyl acetate
Drowsiness and dizziness if inhaled , highly flammable
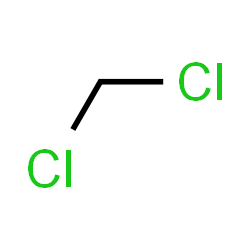
Dichloromethane
Irritating to the eyes and skin
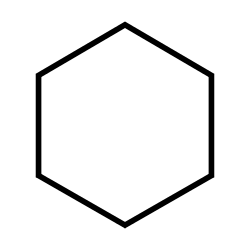
Cyclohexane
Flammable
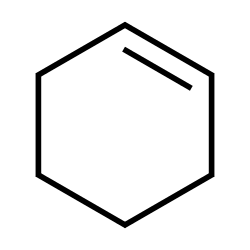
Cyclohexene
Inhalation at high amounts results in narcotic effect
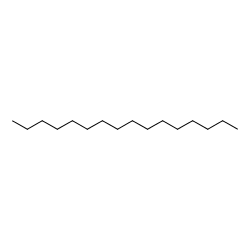
N hexadecane C16H34
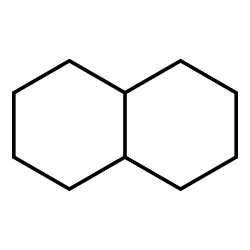
Decalin
Can irritate the skin and eyes, can cause skin rash
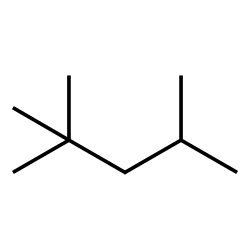
Isooctane
Breathing can irritate the nose, hazard to throat and lungs
B-pinene
Skin irritant
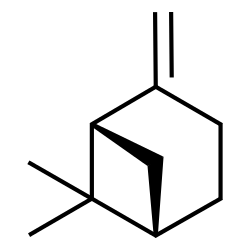
Camphene
Emits flammable vapors when heated
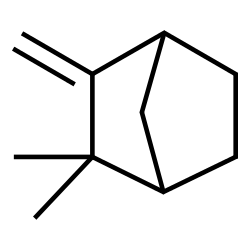
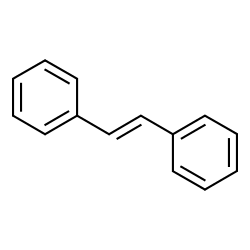
Trans stilbene
Harmful if swallowed
All possible structures for isomers of C6H14
Hexanem 2-methylpentane, 3-methylpentane, 2,2 dimethylpsntane, 2,3-dimenthylbutane
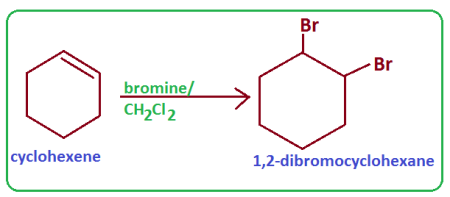
Write equations for Cyclohexene and bromine
Write equation for bromine and 2,2,5- trimethylhexane
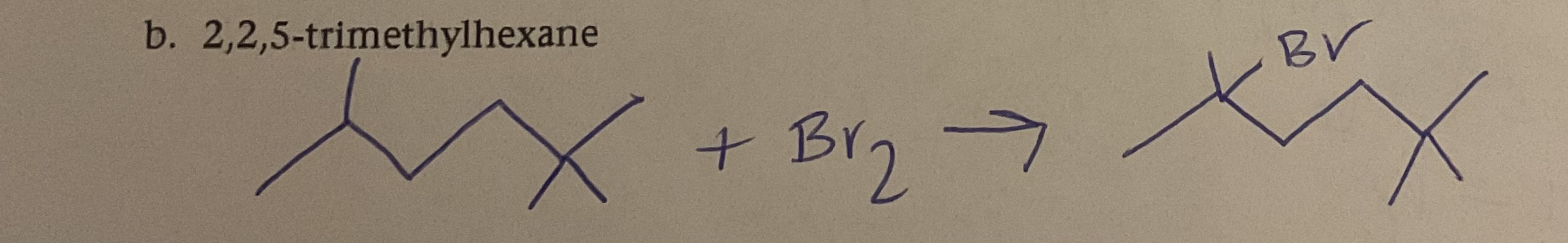
Octane number
The higher the octane number the higher the resistance
Measure of a fuels resistance to knocking during combustion.
Number indicates how well a fuel can resist the premature ignition
Catalytic converter
An emission control device found in the exhaust of most vehicles. It converts toxic gases and pollutants to less harmful substances before they’re released into the atmosphere
Green house effect
Prevent heat produced on hearth from being released. This causes an increase in temperature because of climate change
Allylic bromination
The substitution of bromine for a hydrogen on a allylic carbon
Name any two reaction mechanisms that saturated hydrocarbons use in their reactions
Substitution reactions: one or more atoms or groups of atoms in the hydrocarbon are replaced, common for alkanes
Combustion reactions: carbon can undergo C.R with an oxygen present
How do BHA and BHT accomplish this task?
They accomplish this by donating hydrogen atoms to neutralize free radicals and interpret the chain reaction of lipid oxidation
What kind of interactions are responsible for the solubilities and insolubilities observed in this experiment
London dispersion forces
Hydrogen bonding
Polar forces
Write the possible monochlorrination products and calculate the percentage of each isomer in the product mixture
3-chloro- 2,2,4,4-tetramethylpentane,
1-chloro2,2,4,4- tetra methyl pentane