Concept 2.1: Matter consists of chemical elements in pure form and in combinations
1/8
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Compound
Two or more elements in a fixed ratio joined by bonds which determine its properties
Protons
The positive subatomic particles in an atom that determine an atom’s identity
Electrons
The negative subatomic particles in an atom that determine an atom’s ability to form bonds
Matter
Anything that takes up space and has mass; this is made up of elements
Element
A substance that cannot be bropken down to other substances by chemical reactions
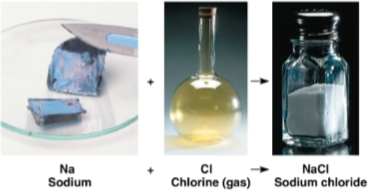
Emergent properties
Characteristics of a compound that are different from those of its elements
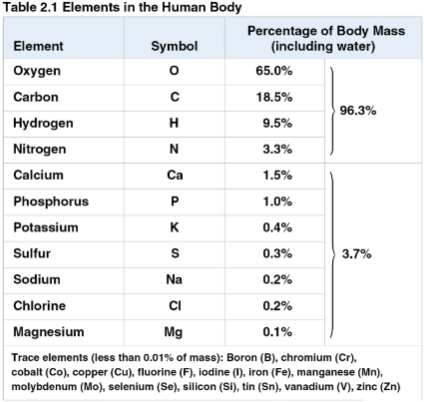
Essential elements
The 20 to 25% of the 92 natural elements required for life
Crabon, hydrogen, oxygen, and nitrogen make up 96% of living matter
The remaining 4% consists of calcium, phosphorus, potassium, and sulfur
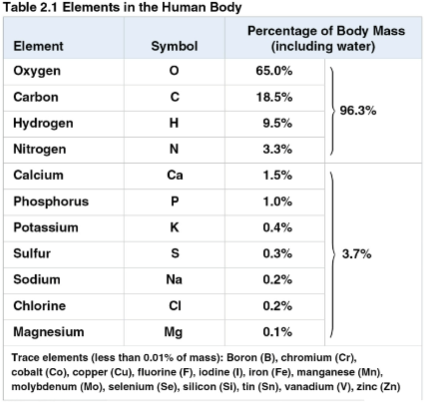
Trace elements
Elements required by an organism in only minute quantities

Toxicity
Some elements can be this; certain species can become adapted to environments containing these elements
Some plant communities are adapted to sepentine