Atoms, Ions, and Isotopes
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
The protons equal the electrons
What determines if the atom is neutral?
electron cloud
Where are the electrons in an atom found?
Protons and Neutrons
What is the nucleus made up of?
5 lost
How many electrons were gained or lost if the ion symbol is Ca+5
One
How many electrons did this atom lose?
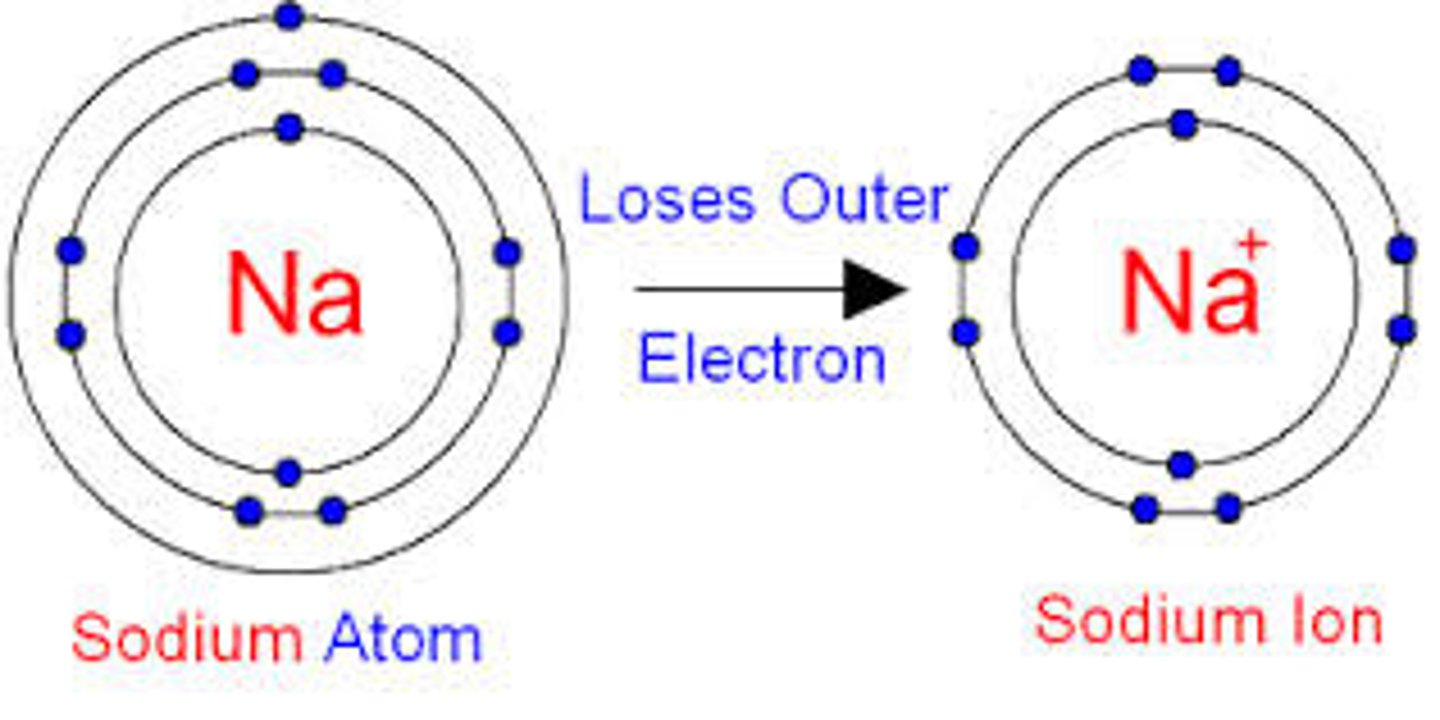
2+
What is the ionic charge of an atom that has lost two electrons?
3+
What is the ionic charge of an atom that has lost three electrons?
4+
What is the ionic charge of an atom that has lost four electrons?
3-
What is the ionic charge of an atom that has gained three electrons?
2-
What is the ionic charge of an atom that has gained two electrons?
1-
What is the ionic charge of an atom that has gained 1 electrons?
negative
An atom becomes a negative ion even though it is gaining electrons because electrons have a ____________ charge.
positive
An atom becomes a _____________ ion even though it is losing electrons because electrons have a negative charge. It is losing its negative charge and becoming more _____________.
Lost
Has this atom lost or gained electrons?
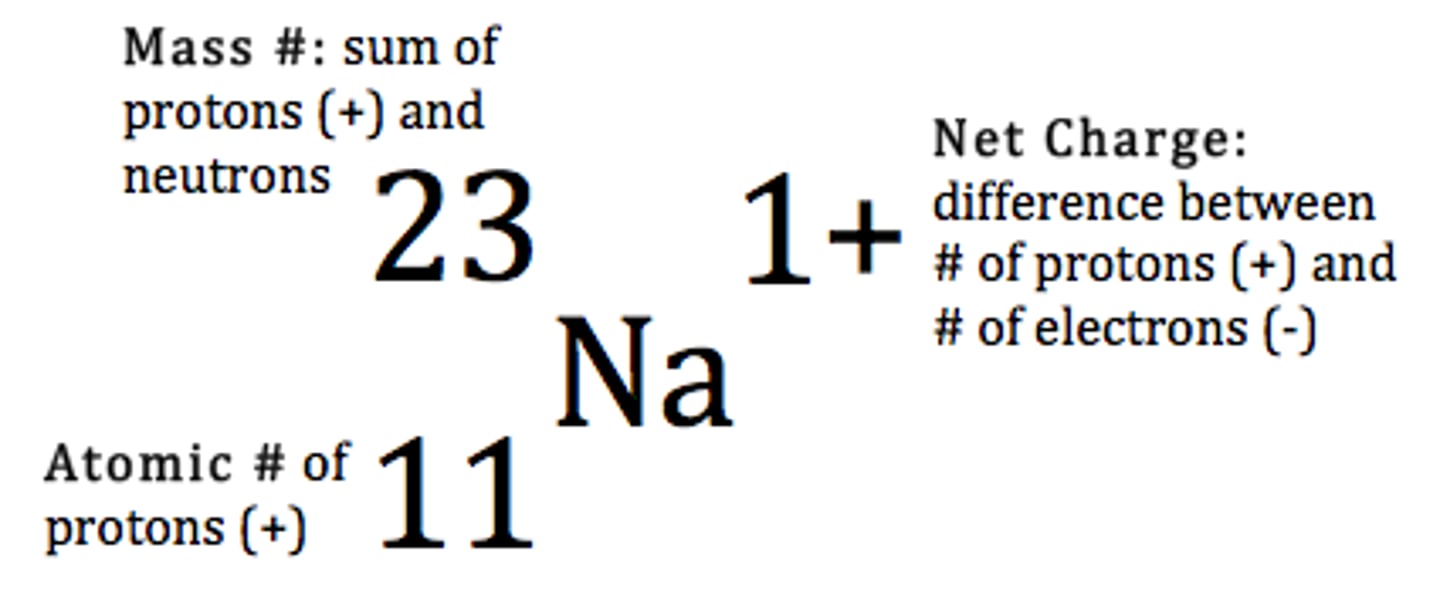
Gained
An ion has a charge of -1. Has it lost or gained electrons?

ion
A particle that is electrically charged (positive or negative)
isotope
Atoms of the same element with the same number of protons (atomic number) but a different atomic mass (number of neutrons).
atomic number
Number of protons in an atom
electrons
In neutral atoms, the number of ____________ is equal to the number of protons
Protons
What type of particle determines the atomic number?
Gained 5
How many electrons were lost or gained by an atom that has a charge of -5
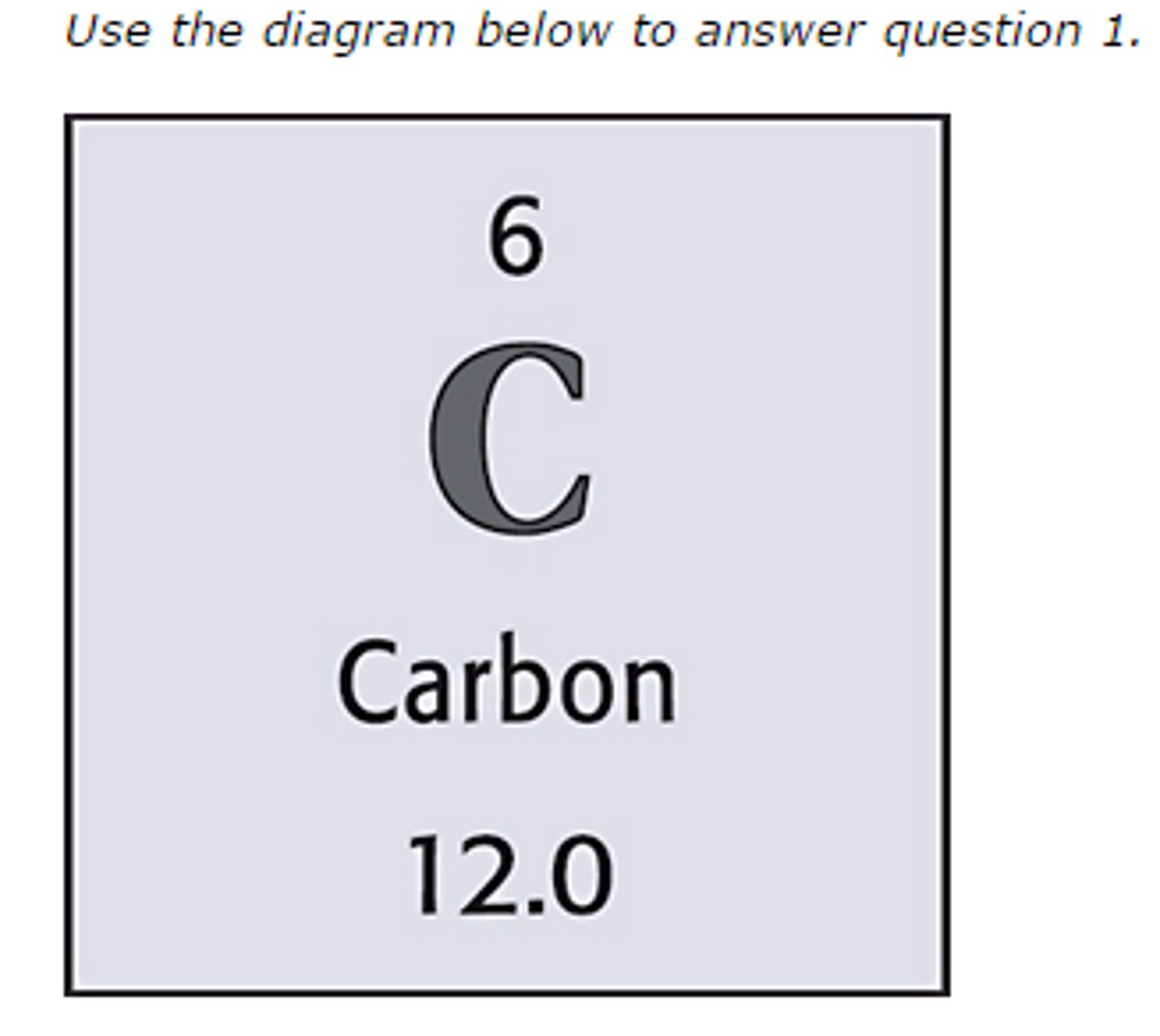
Six
The atomic number of this element
Ten
How many neutrons does the isotope Oxygen-18 have?
Eight
How many electrons does the isotope Oxygen-18 have?
22
How many protons does the isotope Titanium-45 have?