Orgo Chem Quiz #6
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
111 Terms
acids
donate protons
bases
accept protons
conjugate acid
when a base accepts protons
conjugate base
when an acid gives protons
electron movement
making and breaking bonds
curved arrows
describe flow of electron density + physical movement of electrons
same as resonance structures
flow of electron density
base “attacks” acid using electron pair
acid cannot lose its proton without base taking it
shows 2 arrows indicating two pairs of electrons move simultaneously, 1 showing the breaking of the bond and the other showing the building of the bond
pKa
quantitative strength
Ka
acid dissociation constant of an acid dissolved in water
measures acid’s strength when water is a base
Keq
[H3O+] [A-] / [HA] [H20]
Ka
[H3O+] [A-] / [HA]
stronger acid
Ka > 1
weaker acid
Ka < 1
pKa
-log of Ka
stronger acid
lower pKa
weaker acid
higher pKa
order of magnitude
unit of pKa represents this
ex. HBr (pKa = -9) is 10 times stronger acid than HCl (pKa = -8)
weaker
stronger the acid, the _______ its conjugate base is
equilibrium
favors weaker acid + weaker base
products
reactants
subtracting pKa values tells you 10x times more ________ than ________
stability of conjugate bases
without pKa values, we have to evaluate __________
stable
stronger the acid —> more _____ the conjugate base
conjugate base
when acid loses proton, ____________ is formed which contains lone pair of electrons
lone pair
the stability of the conjugate base compares to the stability of the __________
more effectively a conjugate base can stabilize its negative charge/lone pair, the ______ the acid
factors that affect negative charge stability (ARIO)
type of atom
resonance
inductive effects
type of orbital
type of atom
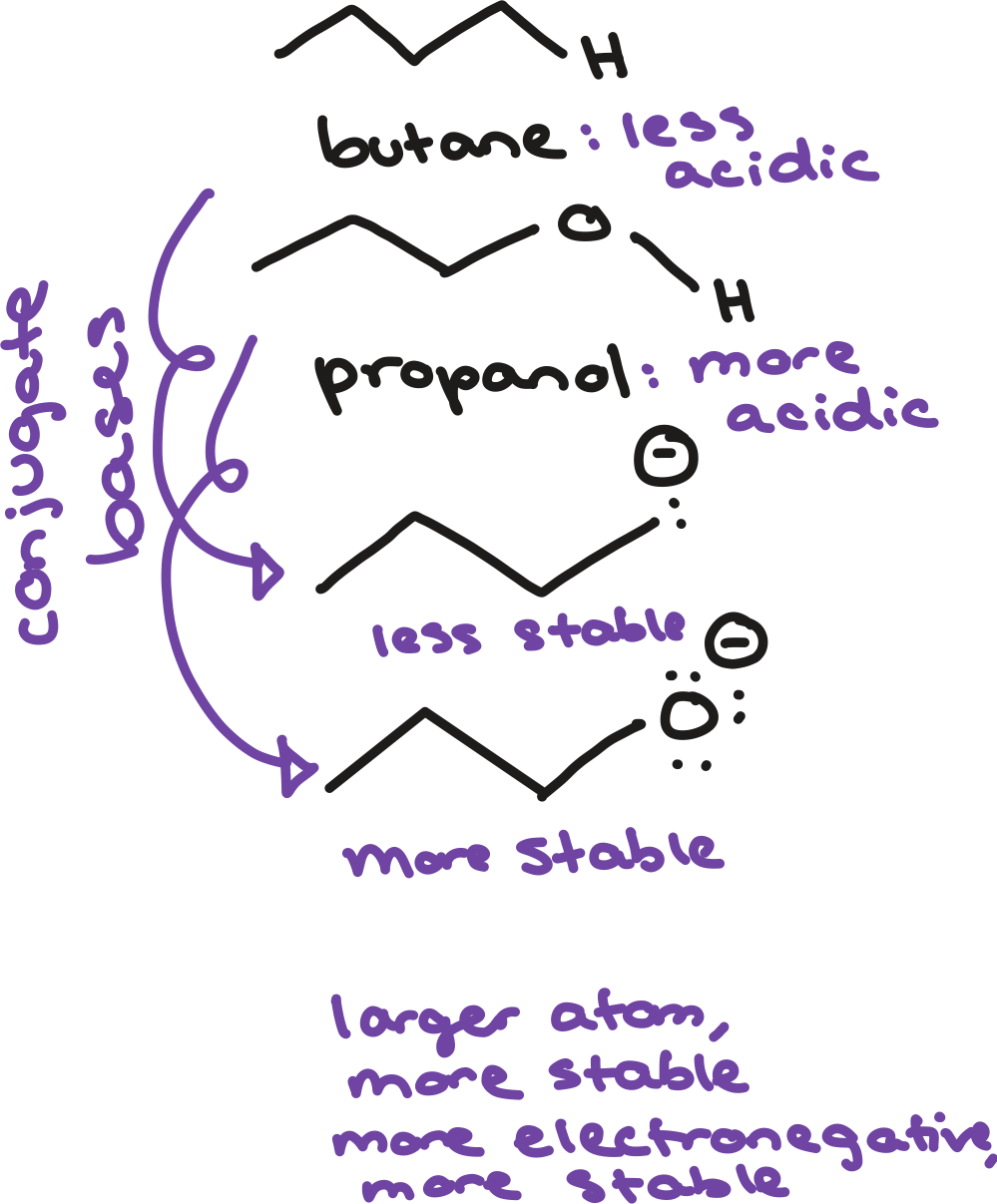
stable
the larger and more electronegative the atom is, the more _____ the conjugate base
stable
the shorter the atomic orbital is, the closer the atom is to the nucleus
the closer the electrons are to the nucleus, the more ____ they are
resonance
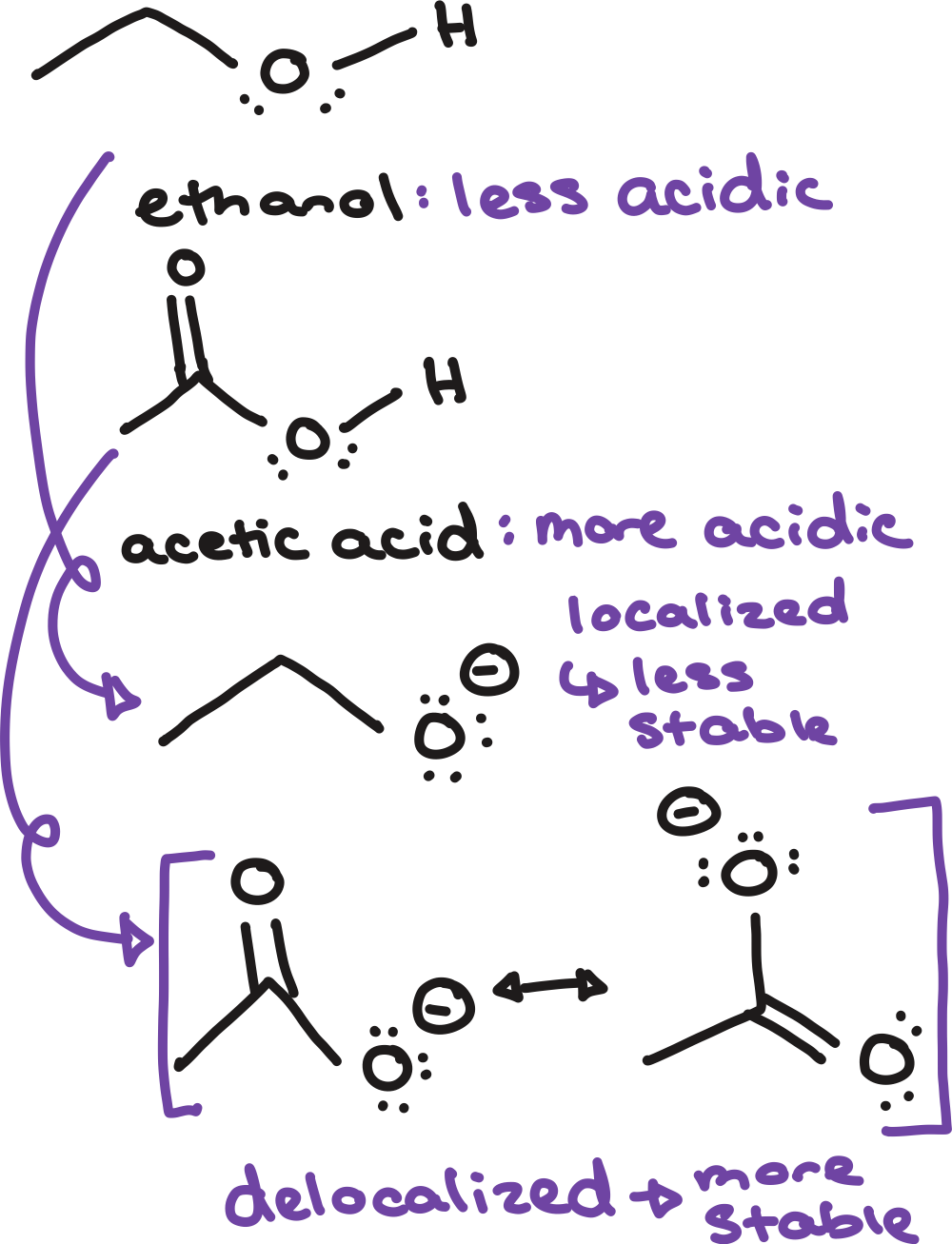
inductive effects
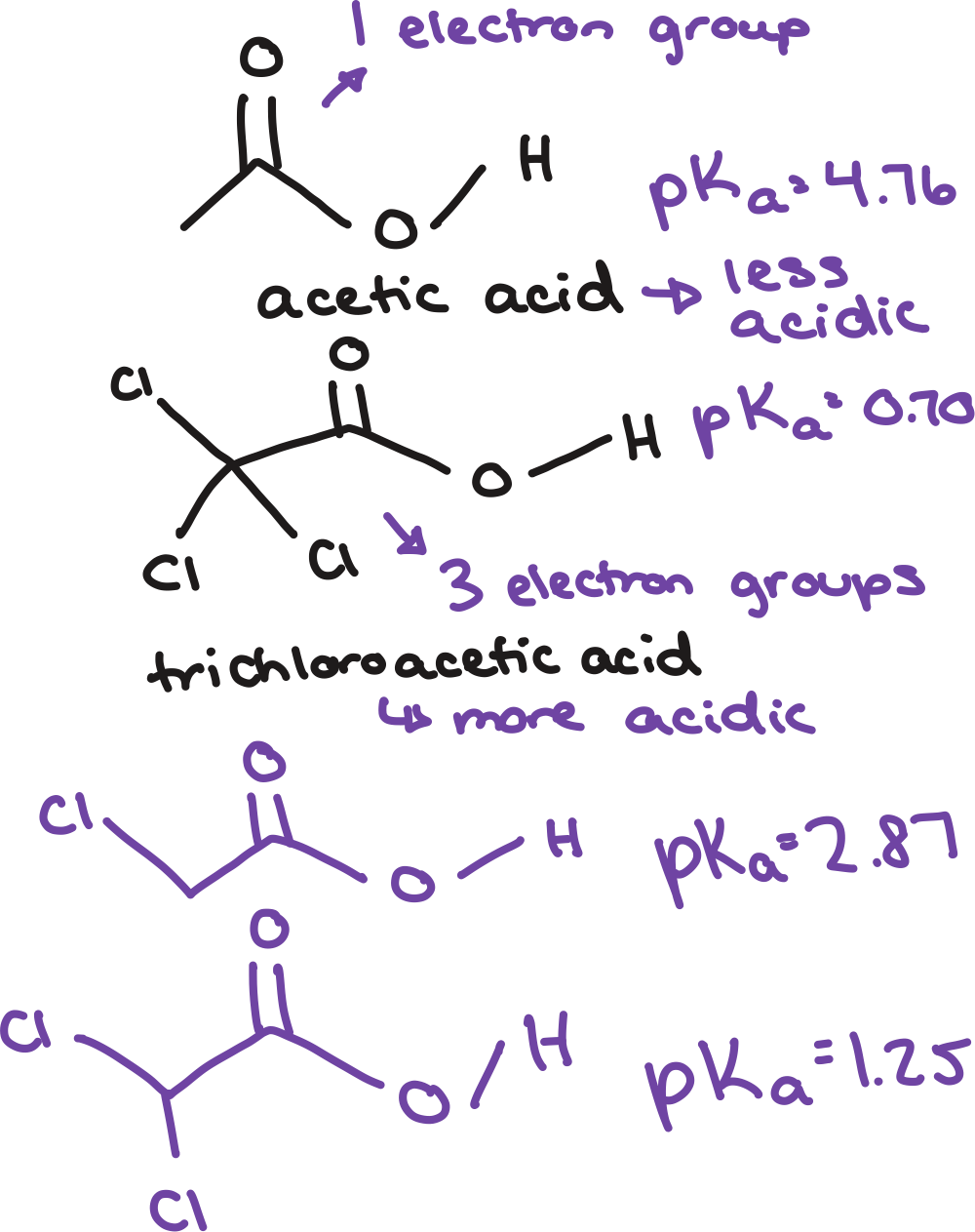
stable
more electron jobs, more ______ conjugate base
stable
closer the electron withdrawing groups to negative charge, more _____ conjugate base
type of orbital
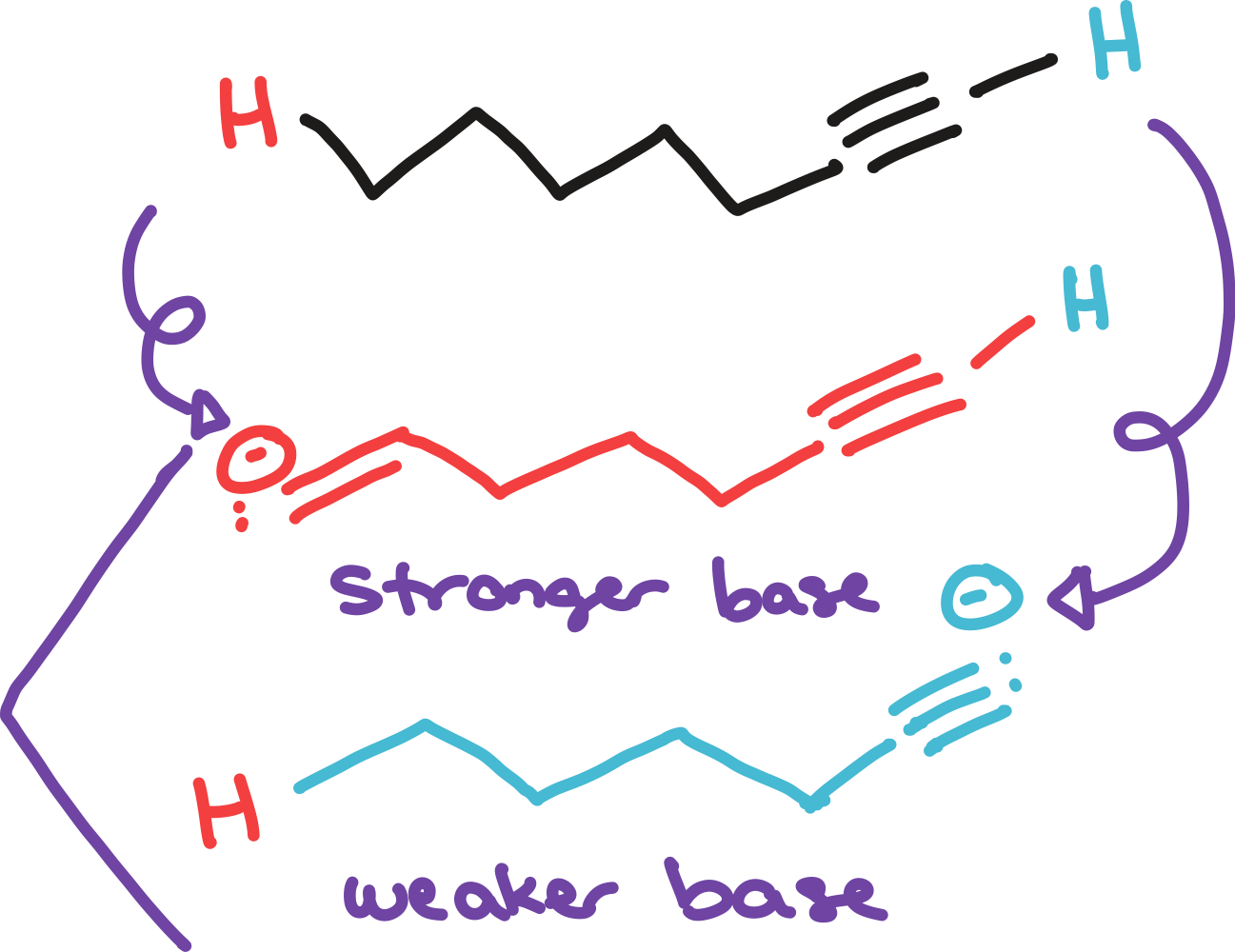
stable
more s-character, more _____ the negative charge
lewis acid
accepts pair of electrons
lewis base
donates pair of electrons
lewis acids and bases
acids under Bronsted-Lowry definitions are also considered _______
bronsted-lowry and lewis acids and bases
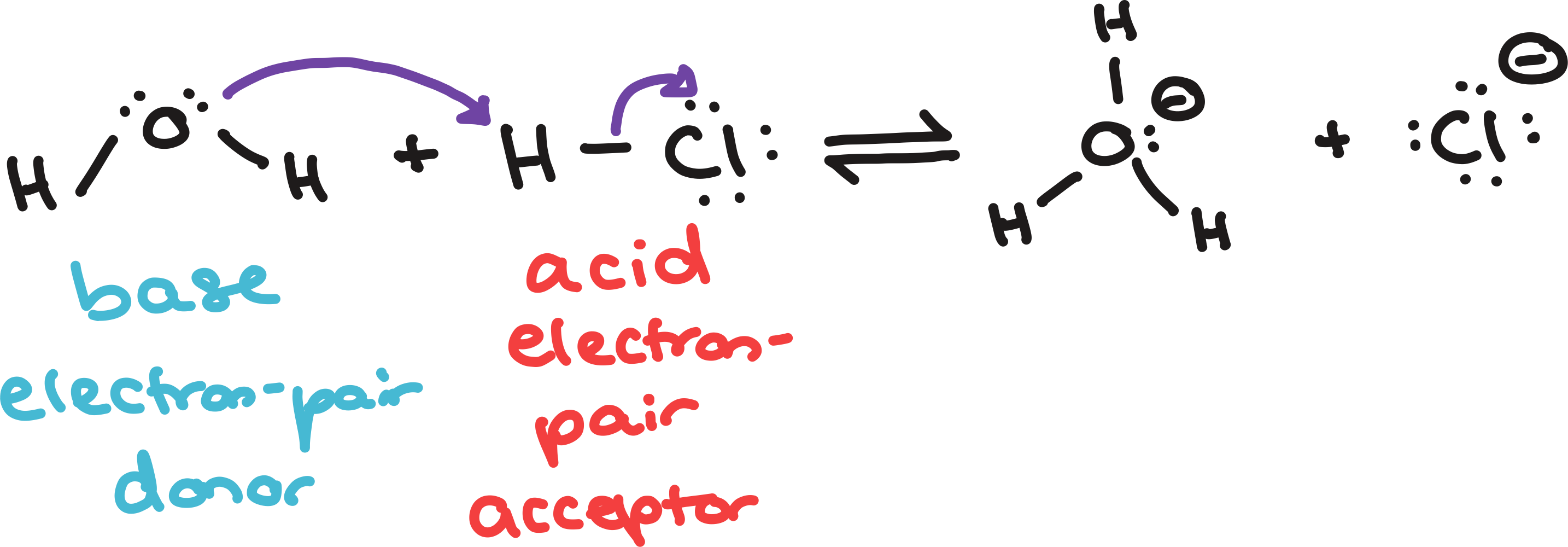
ONLY lewis definitions
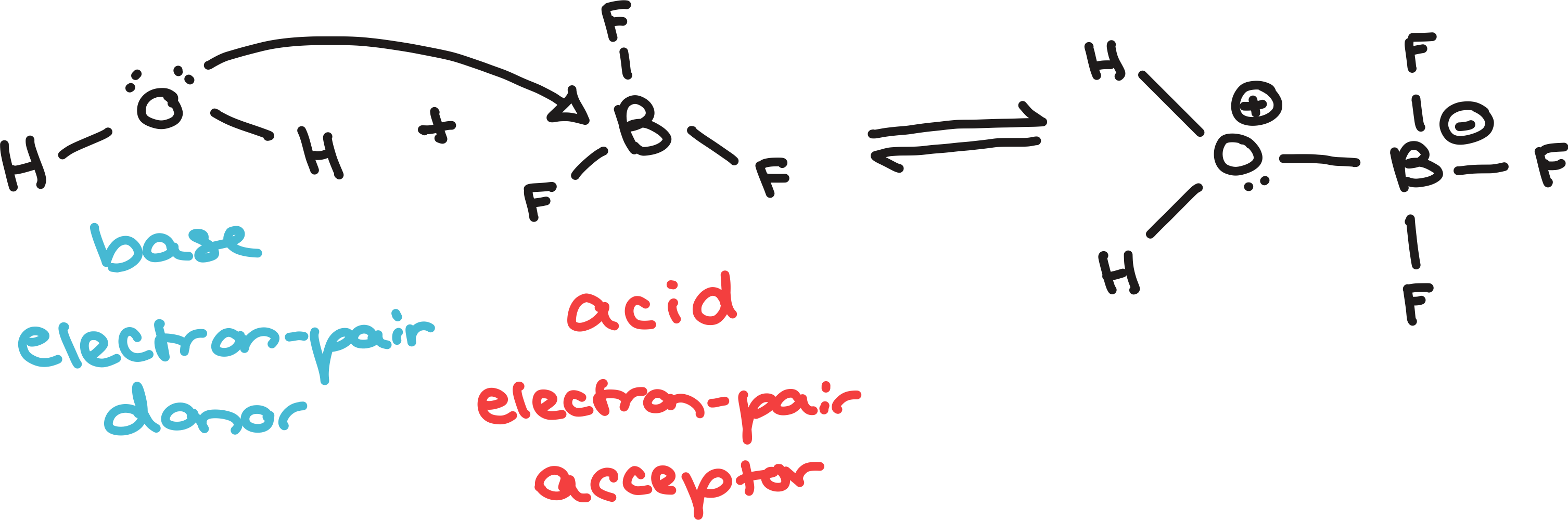
lewis definitions
conjugate acids and bases are not produced since it creates one product
amino acids
contain amino group and carboxylic acid group
form zwitterons (number of positive and negatively charged functional groups) at physiological pH
heat energy
exchange between reaction and surroundings
breaking bonds
system absorbs energy
bond
electrons that absorb kinetic energy to overcome stability of ____
how bonds can break
heterolytically and homolytically
homolytically
produce radicals
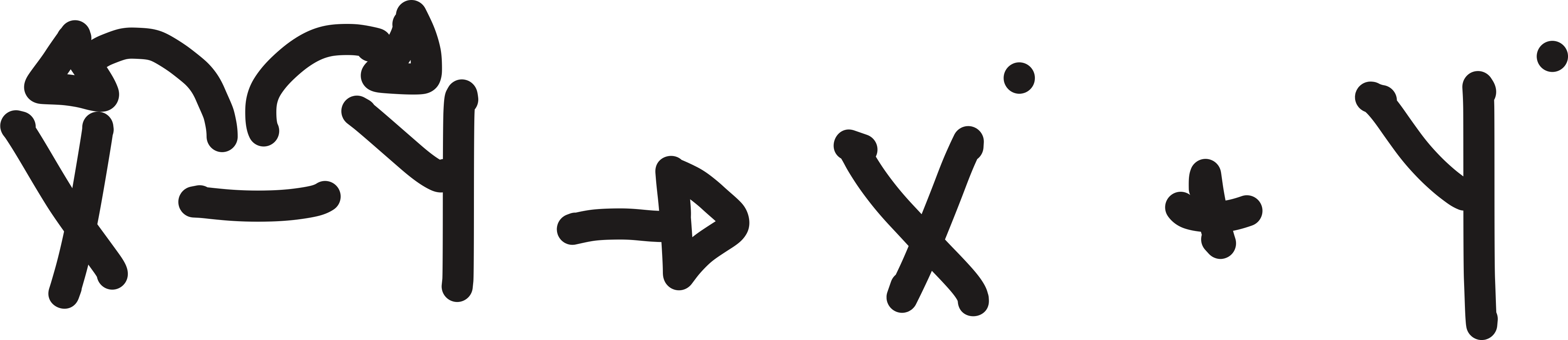
heterolytically
produces ions
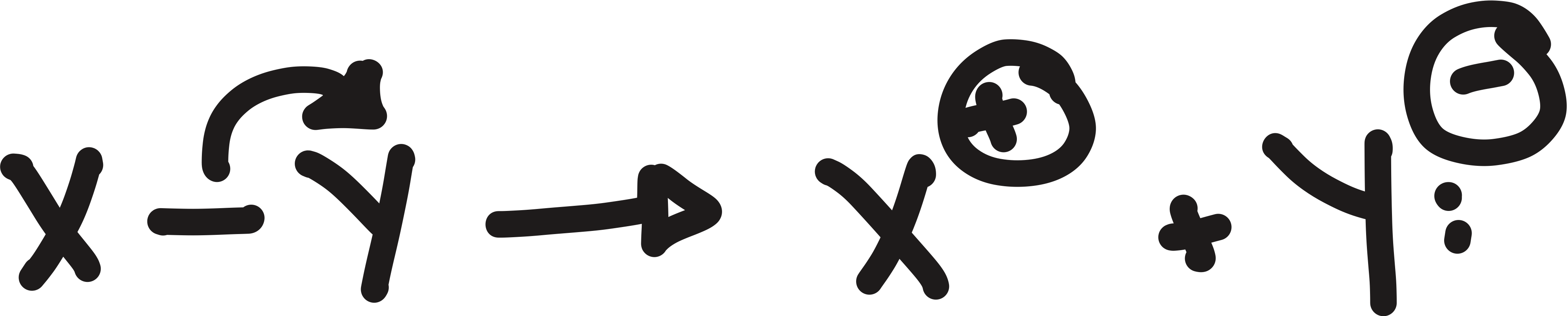
bond dissociation energy (BDE)
homolytic bond cleavage
when bonds break or form
exothermic
energy gained by bonds formed exceeds energy needed for bonds to be broken
products are more stable that reactants
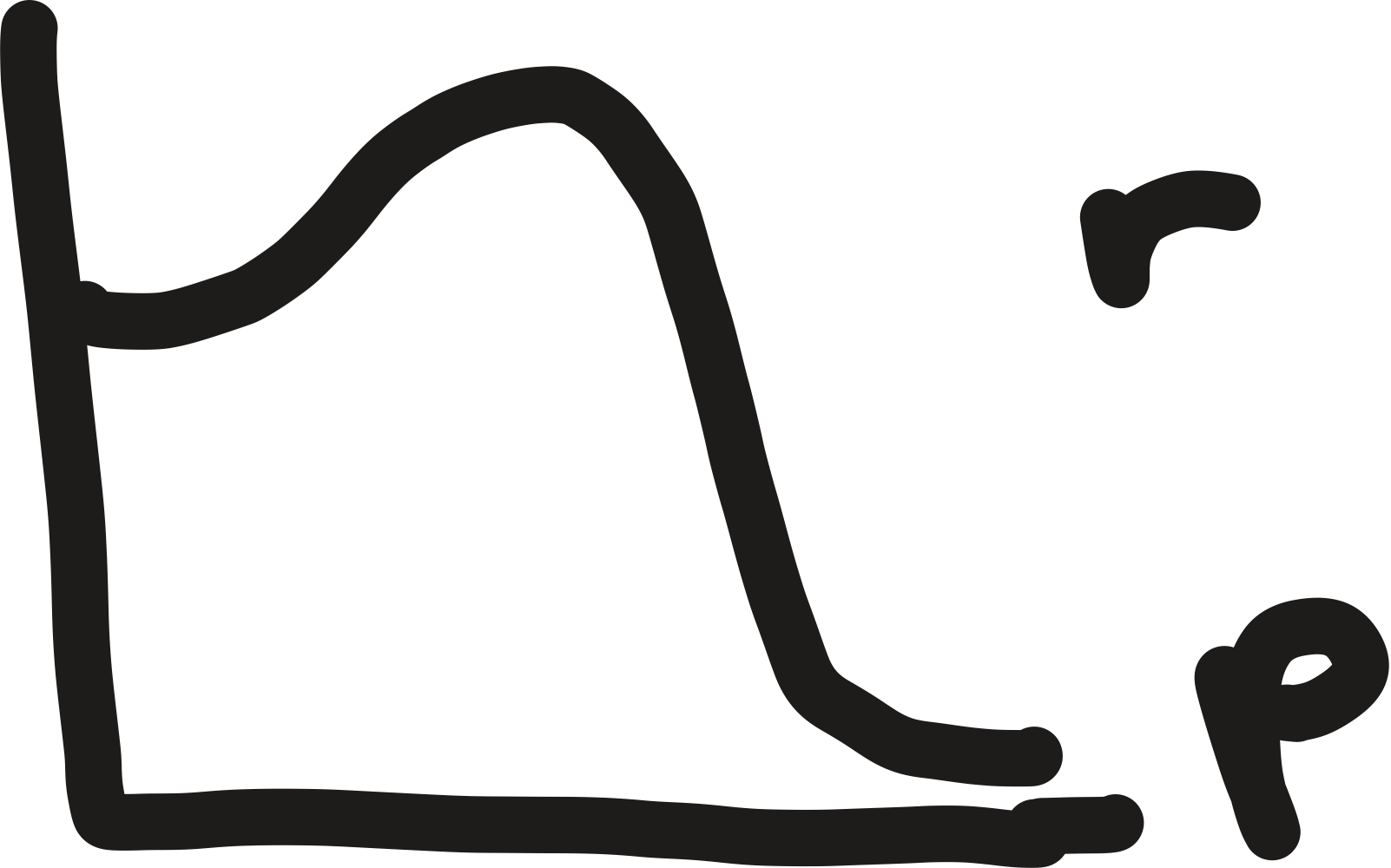
endothermic
energy needed for bonds broken exceeds stability gained by bonds formed
products are less stable than reactants
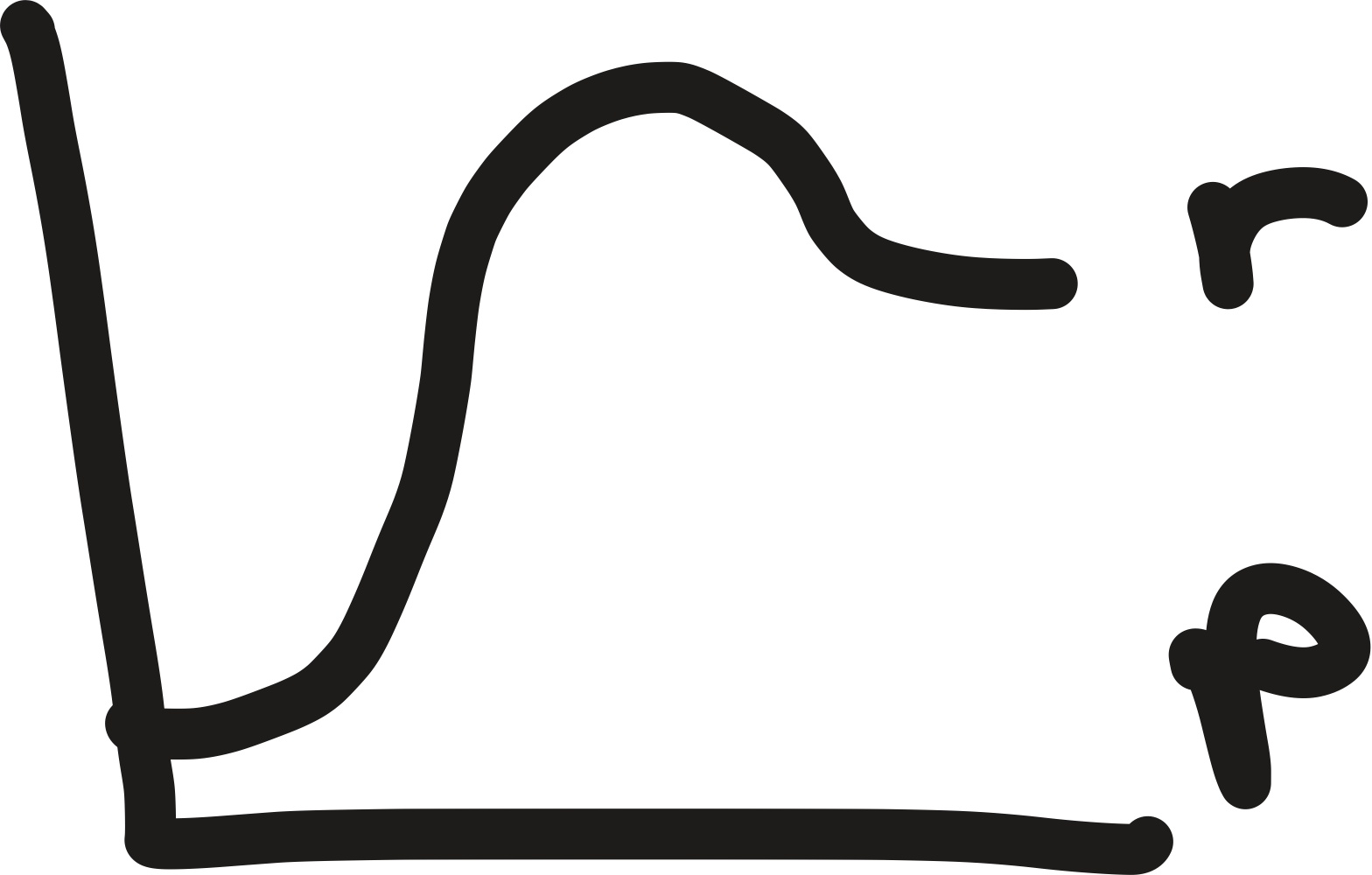
energy diagram
describes kinetics and thermodynamics of reaction
potential energy = y-axis
reaction progress = x-axis
enthalpy (△H)
BDE (bonds broken) - BDE (bonds formed)
entropy
molecular disorder, randomness, or freedom
number of vibrational, rational, and translational states among which the energy of compound is distributed
number of states the molecules spread across increases with increasing volume
enthalpy (△H)
total heat content of system
entropy (△S)
more volume for gas to occupy, ______ entropy
spontaneous
if total entropy is positive, reaction is _______
positive
spontaneous
if there are more moles of product than reactants, system entropy will be ______ and reaction will be ________
spontaneous
positive
if cyclic compound becomes acyclic, reaction will be ______ and system entropy will be _______
spontaneous
exergonic
if △G is negative, reaction is _____ and _______
nonspontaneous
endergonic
if △G is positive, reaction is ______ and _______
△H
change in enthalpy of surroundings
△S
change in entropy of system
Gibb’s free energy (△G)
△H - T△S
products
△G is negative, ______ are favored
reaction rate
function of number of molecular collisions that will occur in a given period of time
reaction rate factors
reactant concentration
activation energy
temperature
geometry and sterics
presence of catalyst
rate law
k[reactants]
rate constant
k
reactant concentration
[reactants]
rate order
represented by x + y variables
first order
rate = k[A]
second order
rate = [A][B]
third order
rate = [A]²[B]
activation energy
energy barrier between reactants and products
minimum amount of energy required for molecular collision to result in reaction
decreases
as activation energy increases, the number of molecules possessing enough energy to react ______
higher
lower activation energy means _____ reaction rate
faster
raising temperature will result in a _____ reaction
more
At higher T, molecules have ____ kinetic energy and ____ molecules will have enough energy to produce reaction
steric considerations
steric hindrance and geometry of compound affect rate of reaction
orientation
when molecules collide, they must have correct ________ for bonds to made/broken
low
if reactive conformation of compound is high energy, it will spend less time in that conformation, so the probability of collision resulting in reaction is ___
catalysts
speed up reactions without consumption
ex. enzymes
lowers activation energy
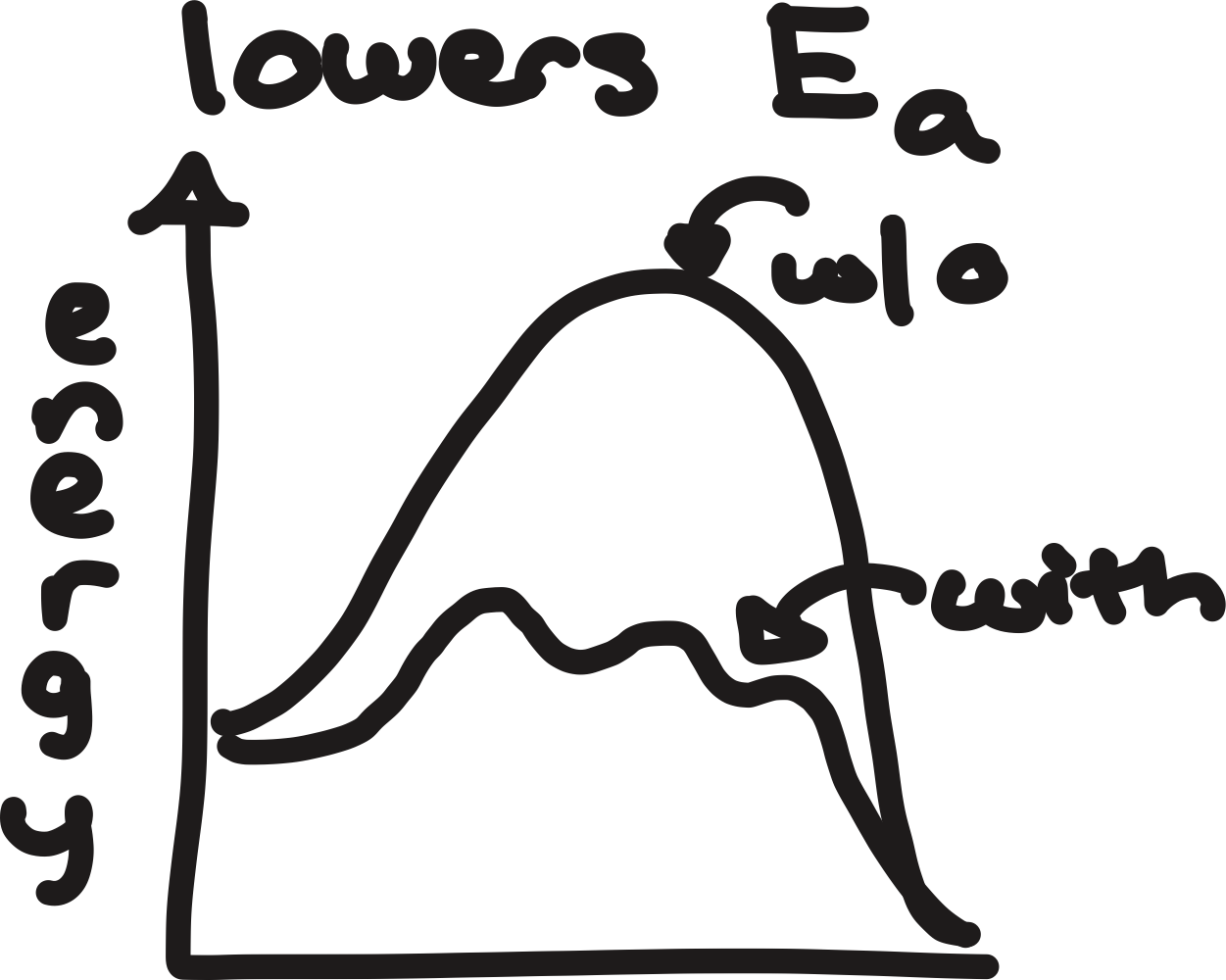
kinetics
____ = reaction rate
thermodynamics
_______ = equilibrium
kinetically favored products
lower activation energy and products are lower in energy, form faster, and are more stable
higher activation energy
higher activation energy and products are higher in energy, form slower, and are less stable
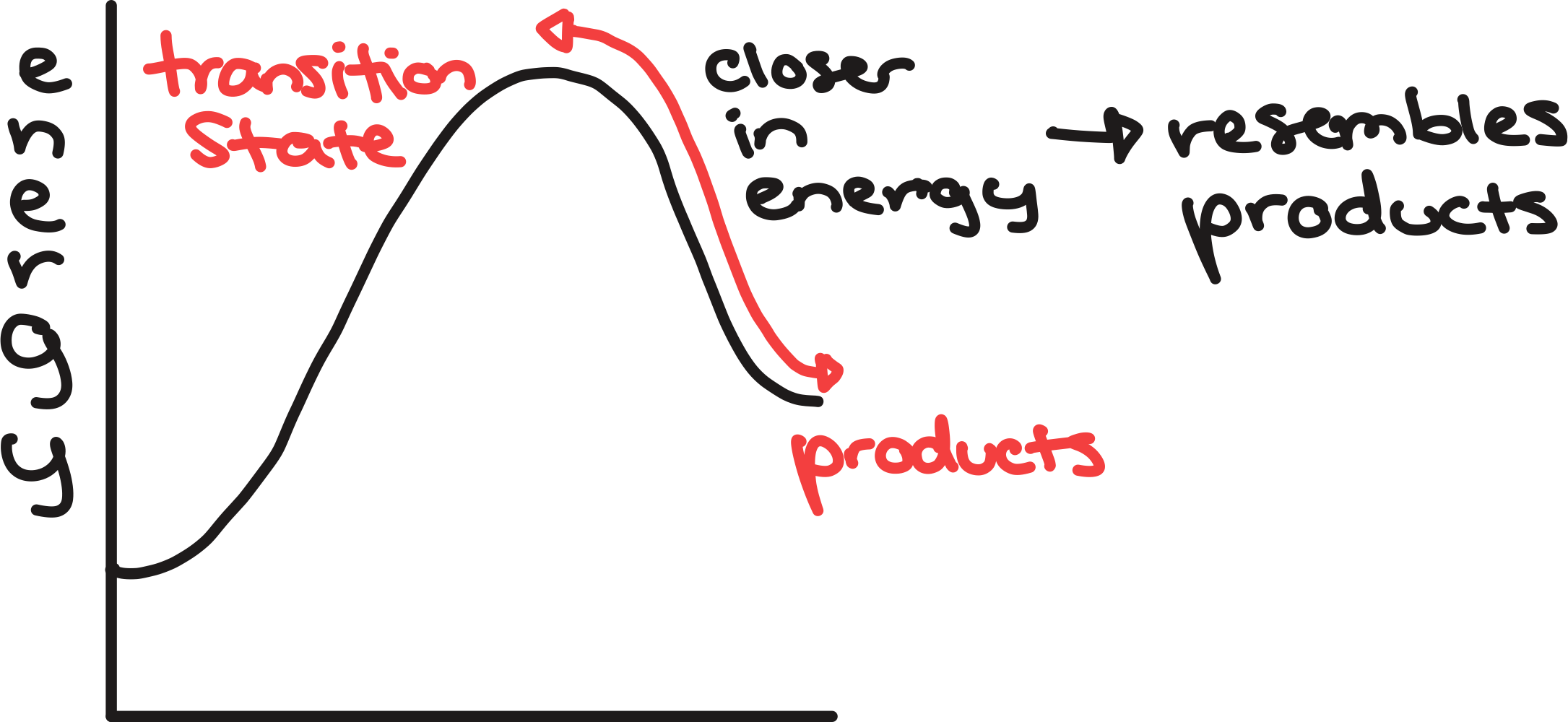
transition state
high-energy state a reaction passes through
energy maxima → represent the transition as bonds being made and broken
not observable
intermediate
species during reaction
exist for period of time
observable
2 points on diagram that are close in energy
similar structure
can generalize the structure of transition state depending on if reaction is exo- or endothermic
exothermic reaction
transition state resembles reactants on diagram
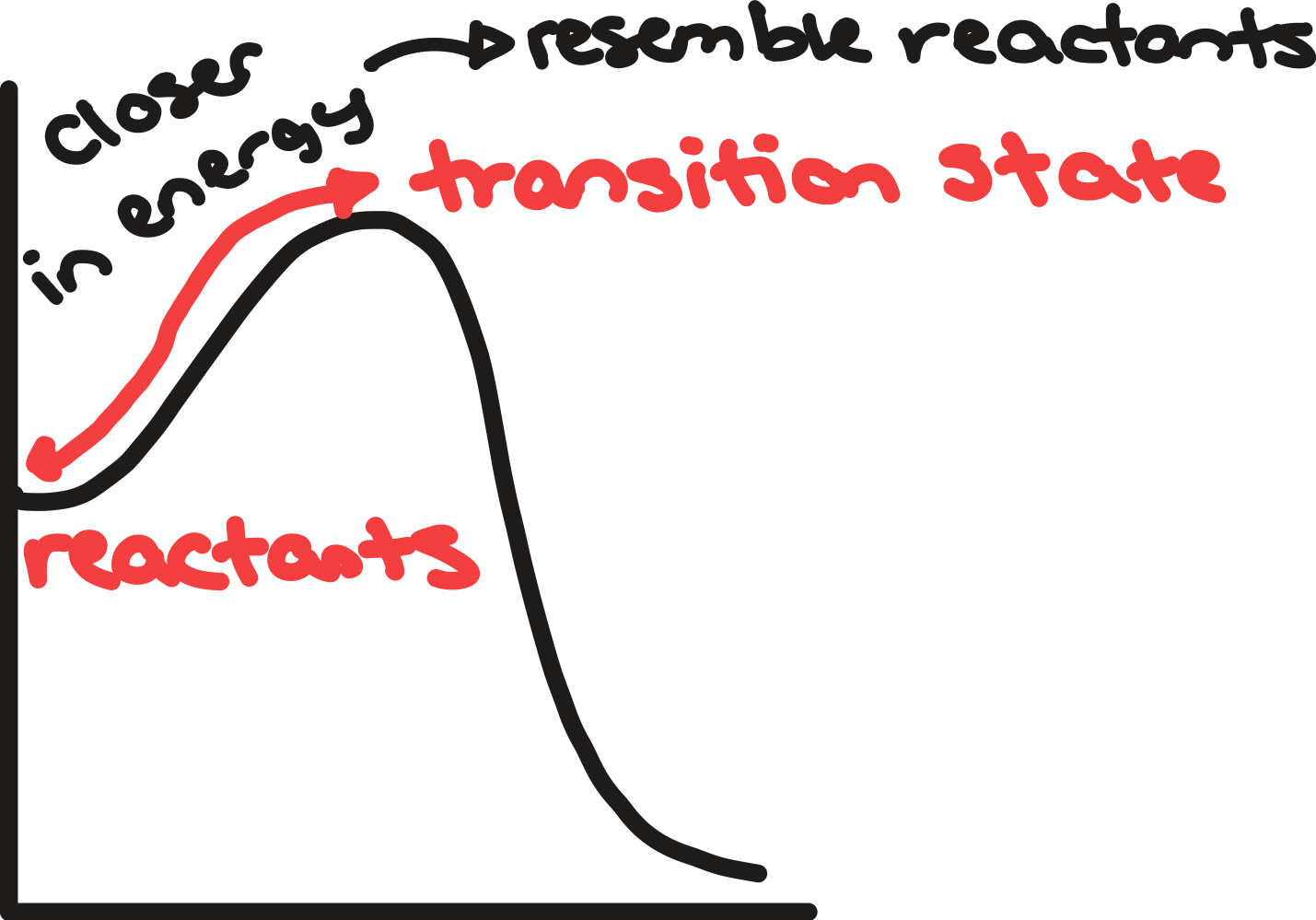
endothermic reaction
transition states resemble products on diagram
polar reactions
involve ions as reactants, intermediates, and/or products
negative attracts positive
electron-rich species attract electron-deficient species
nucleophile
electron-rich, donate electron pairs
lewis base
nucleophile
more polarizable, the stronger the _______
electrophile
electron-deficient species, accept electron pairs
lewis acid
ex. carbocations and partially positive atoms
curved arrows
show how electrons move as bonds break and form

patterns of polar reactions
nucleophilic attack
loss of leaving group
proton transfer
rearrangements
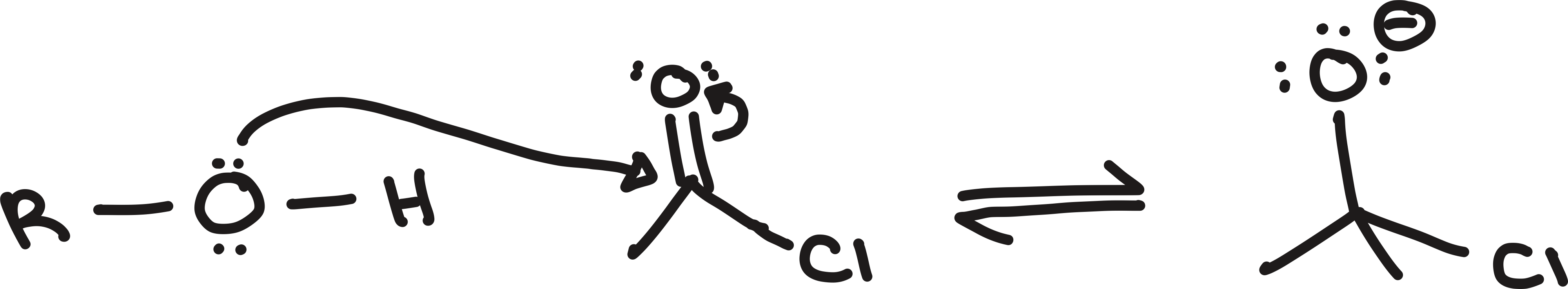
nucleophilic attack
nucleophile attacking an electrophile
tail of arrow starts at negative charge
head of arrow ends on positive charge
electrons end up being shared rather than transferred
may need more than 1 arrow
nucleophile
pi bonds act as _______