cytokines, chemokines and hypersensitivity reactions
1/47
Earn XP
Description and Tags
week 6 clinical immunology
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
cytokines
small proteins released in response to an activating stimulus via specific receptors
many cytokines are called interleukin
different to hormones as:
function relates to immune system (immunomodulating agens)
concs change by many orders of magnitude
most act over a short distance
may be produced by and act on a variety of cells
a broad range of cells can produce cytokines
all immune cells are responsive to at least some cytokines
cytokines may be produced by more than one type of cell
cytokines act through receptors
cytokines may act in autocrine, paracrine and endocrine mofes
all nucleated cells can produce type I interferon
endothelial cells are particularly sensitive to inflammatory cytokines as they regulate vascular adhesion and permeability
cytokine mode of action
autocrine: a cytokine may bind via receptors of the same cell that secreted it
paracrine: a cytokine may bind to receptors on target cells near the cell that secreted it
endocrine: a cytokine may target cells in distance parts of the body
cytokines induce the acute-phase response
TNF-α, IL-1β and IL-6 activate hepatocytes to synthesise acute-phase proteins and activate bone marrow endothelium to release neutrophils
these acute phase proteins act as opsonins
TNF-α, IL-1β and IL-6 are endogenous pyrogens, raising body temp
induce synthesis of prostaglandin E2, which acts on the hypothalamus altering the body’s temperature regulation and on muscle and fat cells
alter energy mobilisation to increase body temp
biological effects of cytokines
pleiotropy: cytokine has different effects on different target cells
redundancy: >2 cytokines mediate similar functions
synergy: combined effect of 2 cytokines is greater than the addictive effect of the individual cytokines
antagonism: 1 cytokine inhibits or offsets the effects of another cytokine
cascade induction: action of 1 cytokine on a target cell infuces the cell to produce 1 or more other cytokines which in turn may induce other target cells to produce other cytokines
cytokines can be grouped by structure into families
cytokine receptors belong to families of receptor proteins, each with a distinctive structure
heterodimeric class I cytokines receptors have an α chain that often defines the ligand specificity of the recepto
they may share with other receptors a common β and γ chain that confers the intracellular signalling function
may cytokine receptors signal through the JAK-STAT pathway
haemopoietin superfamily of cytokines
includes erythropoietin and interleukins (IL-6) and GM-CSF
IL-6 is released by activated macrophages and modulates the immune response, can trigger fever- active phase reponse and neutrophil production from bone marrow
IL-6 doesn’t activate endothelial cell expression of adhesion molecules or trigger overt inflammation
receptors are tyrosine kianse-associated receptors that form dimers when their cytokine ligand binds
dimerisation initiates cellular signalling from the tyrosine kinases associated with the cytoplasmic domains of the receptor
haematopoietin receptors all signal through the JAK-STAT pathway
IL-1α is a damage associated molecular pattern (DAMP)
IL-1α is released by damaged or necrotic cells
IL-1α is not active in the native state (proteolytic cleavage outside the cell)
functions as an alarmin
active IL-1α binds the IL-1 receptor and triggers inflammation in the same way that IL-1β does
dual function cytokine
nuclear localisation/TF activity
signal transduction
IL-1β requires caspase activation
specific inhibitors of caspase-1 reduce the secretion of mature IL-1β, while precursor IL-1β accumulates inside the cell
primary stimulus increases expression of inactive pro- IL-1β
inflammasomes are activated by NLRPs, resulting in caspase-1 activation
caspase-1 cleavage of pro-IL-1β yields mature, active IL-1β
IL-1β leaves the cell to trigger inflammation in neighbouring cells
TNF family
>17 cytokines
many members are transmembrane proteins- homotrimers
TNF-α, initially expressed as a trimeric membrane bound cytokine but can be released from the membrane
TNF receptor I (TNFR-I) is expressed on a wide range of cells including endothelial cells and macrophages
TNFR-II is expressed largely by lymphocytes
signalling uses members of the TRAF family to activate the non canonical NFκb pathway
TNF-α is a potent inflammatory cytokine
produced mainly by activated macrophages
in lower quantities by some CD4+ T cells, NK cells, neutrophils, mast cells and eosinophils
TNF-α is initially expressed as a transmembrane homotrimer, which can be cleaved by the protease TNF-α converting enzyme (TACE) to release soluble TNF-α
able to induce fever, apoptotic cell death, cachexia and the acute phase response
interferon-γ promotes intracellular defences
IFN-γ is produced predominantly by NK cells, Th1 and cytotoxic T cells
IFN-γ promotes:
microbicidal activity of macrophages
Th1 polarisation of CD4+ T cells
increased MHC expression by APCs
class switching to opsonising and complement fixing isotypes
normal inflammatory response
balance between pro- (IL-10, IL-1RA, TGF-β) and anti-inflammatory cytokines (produced hours after production of pro-inflammatory cytokines)
infection stimulates macrophages to release cytokines and chemokines that initiate an inflammatory response
systemic inflammatory response syndrome (SIRS)
SIRS is an inflammatory state affecting the whole body which can lead to shock, disseminated intravascular coagulation and ultimately multiple organ failure
proinflammatory mediators enter systemic circulation, acting on cells of tissues far away from original inflammatory process
sepsis- bacterial infection of the blood
TNF-α triggers local containment of infection but induces shock when released systemically
local release of TNF-α vs systemic release
in both cases, TNF-α acts on blood vessels
↑ blood flow
↑ vascular permeabilioty to fluid, proteins and cells
endothelial adhesiveness for leukocytes and platelets
blood clots prevent spread of infection via blood, accumulated fluid and cells drain to regional lymph nodes, initiates adaptive immune response
systemic infection or sepsis with bacteria: TNF- α is released into blood by macrophages in the liver and spleen, acts in a similar way on all small blood vessels in the body
result is shock, disseminated intravascular coagulation with depletion of clotting factors and consequent bleeding, multiple organ failure and death
chemokines are a subset of cytokines
chemoattractant cytokines recruit cells along a chemotactic gradient
heparin-binding proteins
homeostatic (constitutively produced in some tissues to regulate leukocyte trafficking)
or
inflammatory (attracting immune cells to the site of inflammation)
4 classes of chemokines
classification based on the locations of the cysteine residues that yield structural disulphide bonds (CXC (α), CC (β), CX3C, C)
many CC and CXC chemokines play a role in inflammation
CC chemokines (β) tend to recruit monocytes
CXC chemokines (α) tend to recruit neutrophils
CC chemokines: monocyte chemoattract protein-1) MCP-1 or CCL2 induces monocytes to leave the bloodstream and enter the surrounding tissue
the only CX3C chemokines discovered to date is CX3CL1
cytokine receptors fall within five families
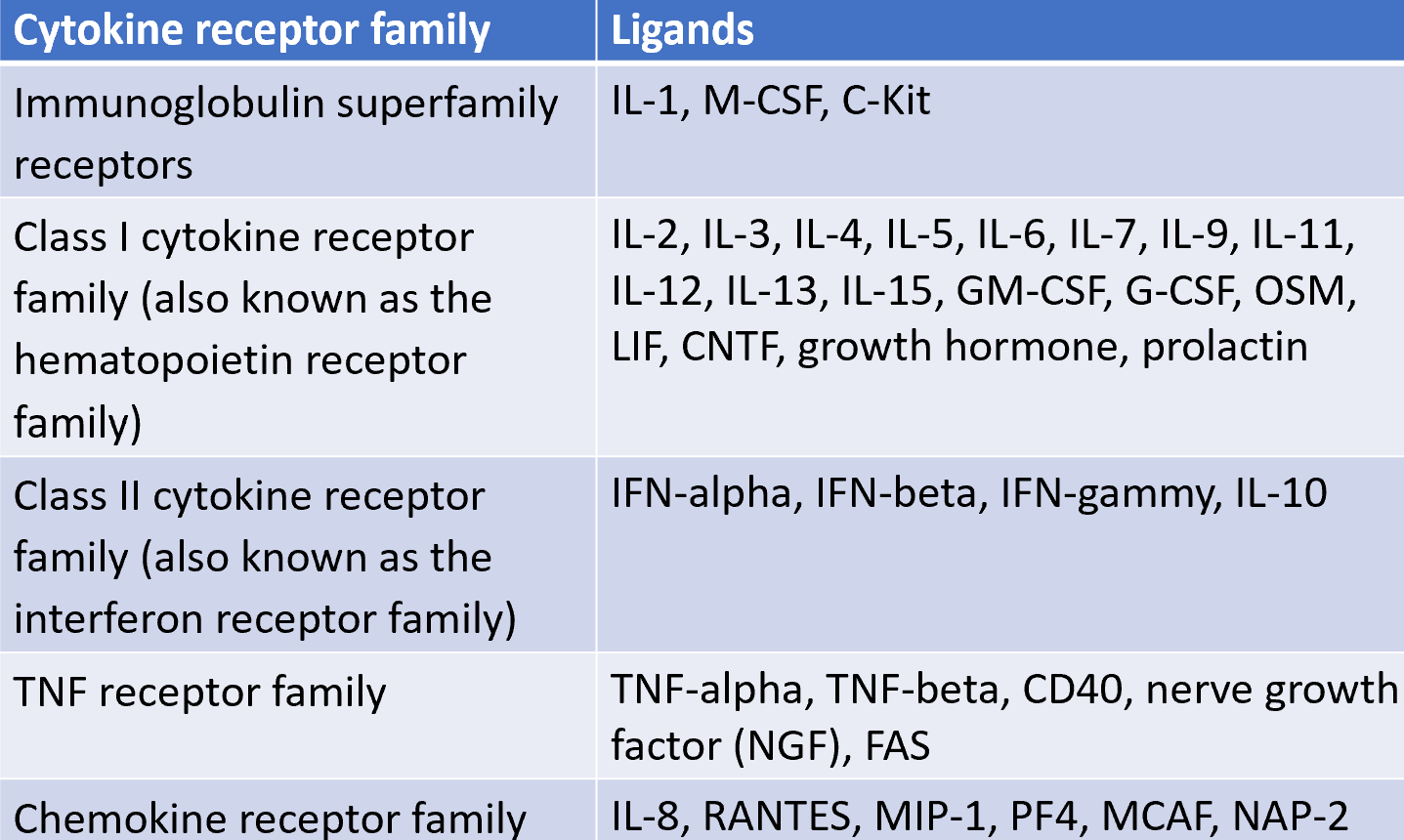
IL-2R
class 1: conserved cysteines (double black lines) and WSXWS motifs (red lines)
IL-2R is trimeric:
α chain: only expressed by activated T cells (also called TAC: T cell activation antigen)
β and γ chains belong to the class I cytokines receptor family (CCCC, WSXWS)
IL-2R occurs in 3 forms
the forms have different affinities for IL-2
activated T cells express 5×103 high affinity receptors
NK cells: consitutively expressed β and γ chains provide intermediate IL-2 affinity and subsequent activation
cytokine receptor
cytokine receptors of the haematopoietin superfamily are associated with the JAK family of tyrosine kinases, which activate STAT TFs
JAK (janus kinase): 2 tandem kinase-like domains
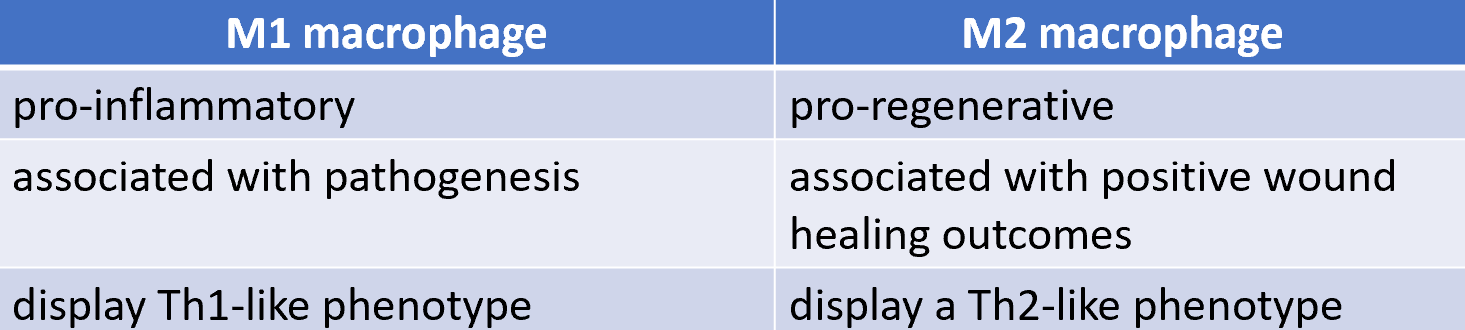
macrophage polarisation
the process by which undifferentiated macrophages produce distinct functional phenotypes as a reaction to specific microenvironment stimuli and signals
classically activated M1 macrophages
alternatively activated M2 macrophages
M1 and M2 macrophages
TH1 cells are the principal T cell helpers for macrophages
some pathogens are not killed by macrophages
peptides derived from such microorganisms can be displayed by MHC class II molecule to TH1 cells
TH1 cells synthesise membrane-associated proteins and secrete cytokines that enhance the macrophages antimicrobial defences
classical macrophage activation: M1 macrophage
M1 macrophage (activated byt Th1 cells)
activated macrophages increase expression of CD40 and of TNF receptors and secrete TNF-α
CD40 ligand and TNF-α synergise with IFN- γ secreted by Th1 cells to induce classicial or M1 macrophage activation whhich is characterised by production of NO and superoxide
B7 molecuoles are upregulated in response to binding CD40L and TNF-α
increased MHC class II expression in response to IFN-γ enables a positive feed-forward loop that enhances activation of Th1 cells
Th2 cells recruit and activate M2 macrophages via IL- 4 and IL-13
macrophages activated by Th2 cells have increased effectiveness in eradicating helminths and promote tissue repair responses
upregulated expression of IL-4 and IL-13 receptors
expression or arginase-1 results in secretion of orthinine and proline
M2 macrophages can repress local tissue inflammation through IL-1 receptor antagonist (IL-1RA) and a decoy IL-1 receptor (IL-1RII)
M2 macrophages also produce the anti-inflammatory cytokine IL-10 and TGF-
CD4+ T cells can polarise into 4 major subsets
subsets are elicited by different classes of pathogens
defined on basis of different combinations of cytokines they secrete
IL-2 = universal activator of T cell proliferation
IL-2 acts in an autocrine manner
IL-2 is switched on when memory T cells get activated
lack of IL-2R is the cause of severe combined immunodeficiency
4 major types of hypersensitivity
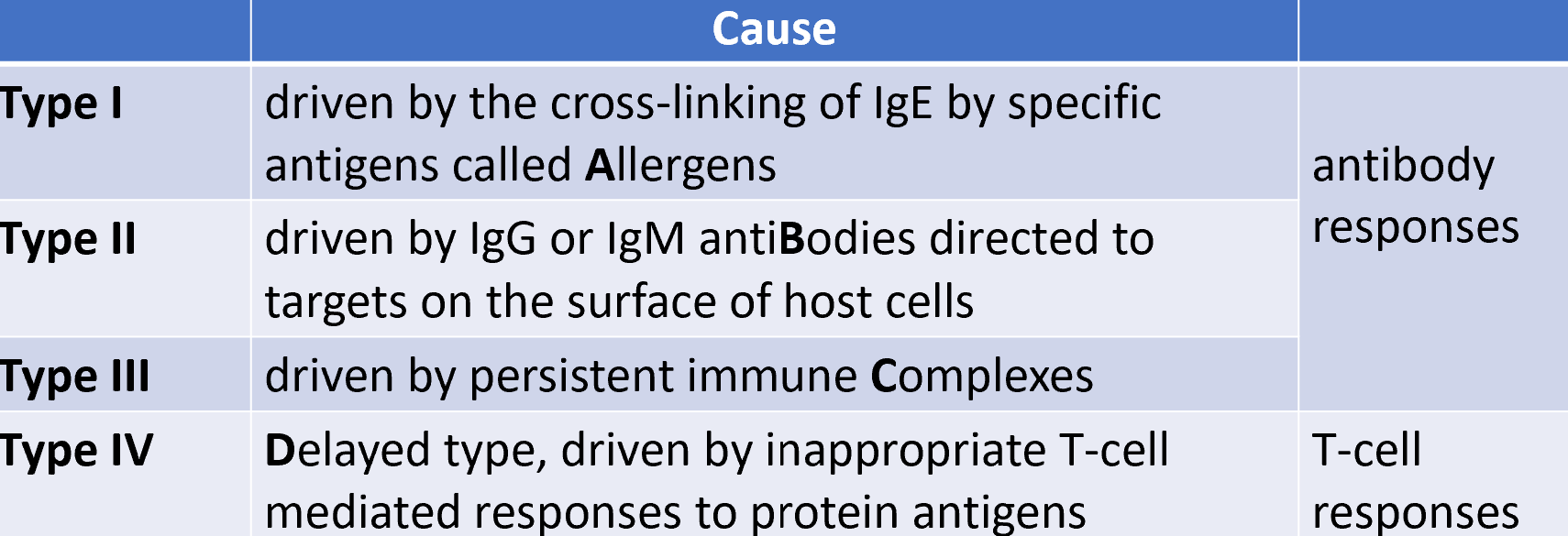
type I hypersensitivity (immediate hypersensitivity)
most common immune disorder
underlying causes of allergy
related consitions include asthma, hay fever
mediated by allergen-specific IgE ABs
class-switching to IgE is dependent on Th2 cytokines
development of type I hypersensitivity
IgE molecules bind to Fc receptors (FcεRI/CD23) on the surfaces of mast cells and basophils
cross-linking of surface-bound IgE molecules generates intracellular signals via CD23
leads to mast cell/basophil degranulation
vascular endothelial cell junctions loosen, increasing vascular permeability with subsequent fluid accumulation in tissues
smooth muscle contraction accelerated fluid distribution from central trunk of body into peripheral tissues
characteristics of type I immediate and late phase reactions
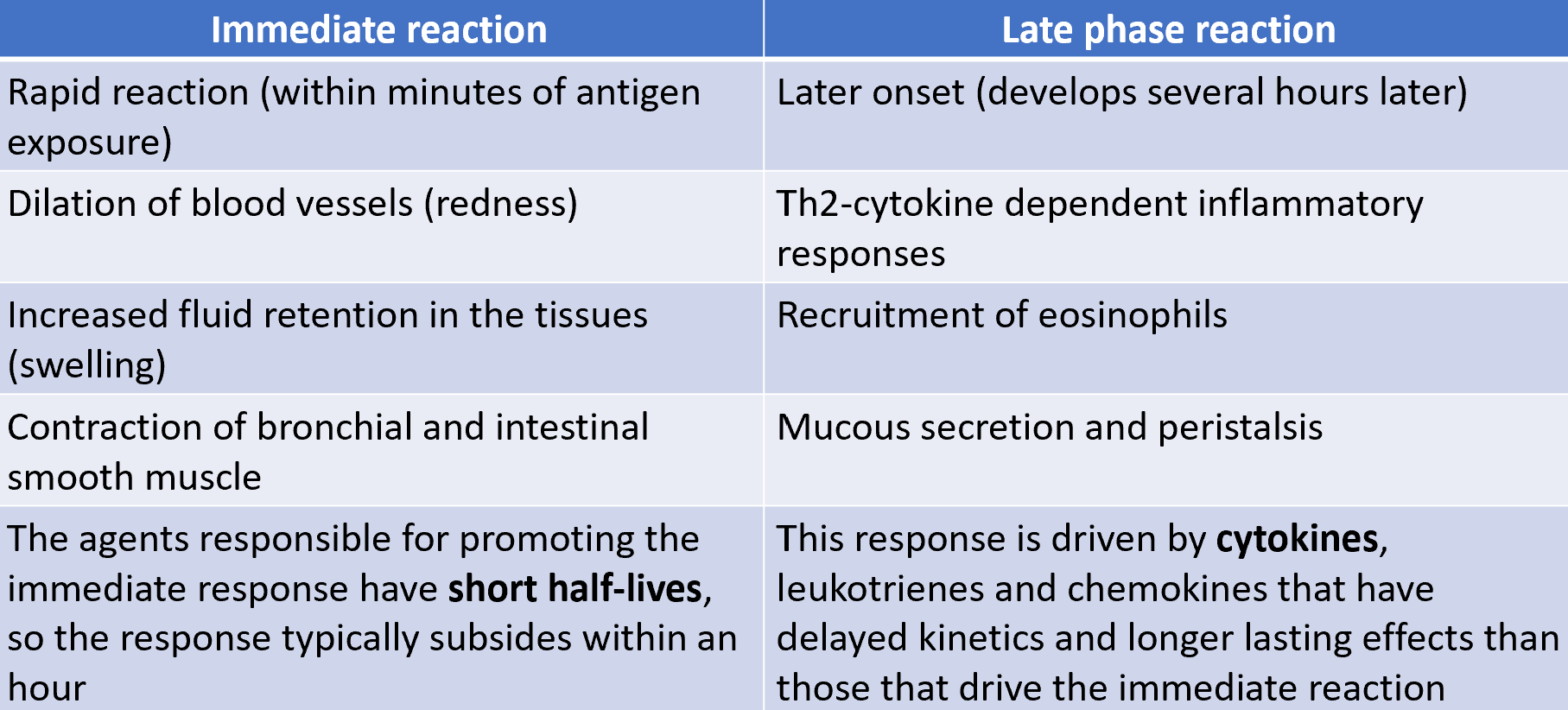
repeated type I hypersensitivity reactions can cause tissue damage
allergic responses can resolve quickly after exposure to allergen leaving little tissue damage
repeated immediate hupersensitivy reactions within tissue can result in tissue remodelling and organ dysfunction
approx 1 in 5 have atopy
anaphylactic shock is an extreme variant of type I hypersensitivity
allergen may be delivered via an insect sting or absorption across intestinal tract
if individual has high traces of IgE, there is a danger of anaphylactic shock
mast cells are activated systemically by circulating antigen
causes vascular dilation and plasma leakage into tissues throughout the body, resulting in low blood pressure
large quantities of mucus may eb secreted into airways, airway smooth muscle contraction caused by mast cell derived mediators may cause difficulty breathing
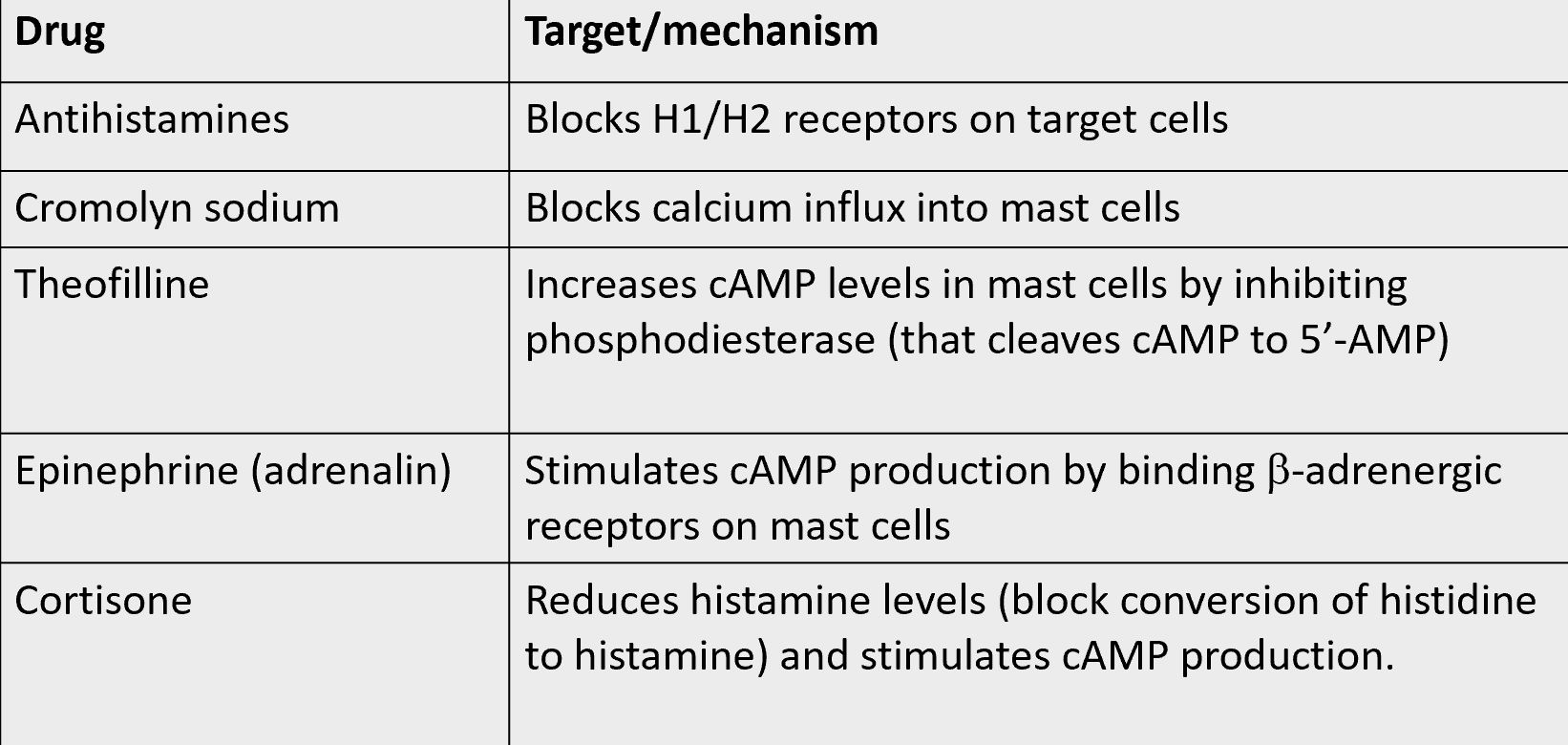
current therapies: adrenaline, anti histamines
mechanism of action used to treat type I hypersensitivity
type II hypersensitivity is mediated by IgM/IgM targeting self antigens
ABs may be generated that bind to self antigens
is target is an immoblile tissue antigen, response is referred to as type II hypersensitivity
pathology is restricted to tissue where the target antigen is expressed
3 mechanisms of type II hypersensitivity:
cells may be damaged by complement activation caused by the cross-linking of ABs
cells may be opsonised by ABs and targeted for phagocytosis
AB dependent celluar cytotoxicity (ADCC) is mediated by IgG ABs bound to antigen on the surface of tagrte cells
recognised by FcYRII on NK cells which degranulate and kill target cell
the binding of cellular receptors by ABs may interfere with their normal function so cause disease
autoimmune haemolytic anaemia (AIHA)
medsiated by IgG (causing phagocytosis of RBC) or IgM (causing complement lysis of RBC) but it’s not clear why these ABs rise
RBCs lifetime reduced to just several days in serious cases
AIHA is generally self limiting in children but can be more serious and require long term immunosuppression
Goodpasture’s Syndrome (GPS) / Anti-Glomerular Basement membrane disease
ABs attack the basement membrane in the lungs/kidneys
target host antigen is the α-3 subunit of type IV collagen
can result in bleeding from the lungs and kidney failure
treatment:
immunosuppressant drugs (corticosteroids)
plasmapheresis (ABs are removed from the circulation)
diseases caused by type III hypersensitivity
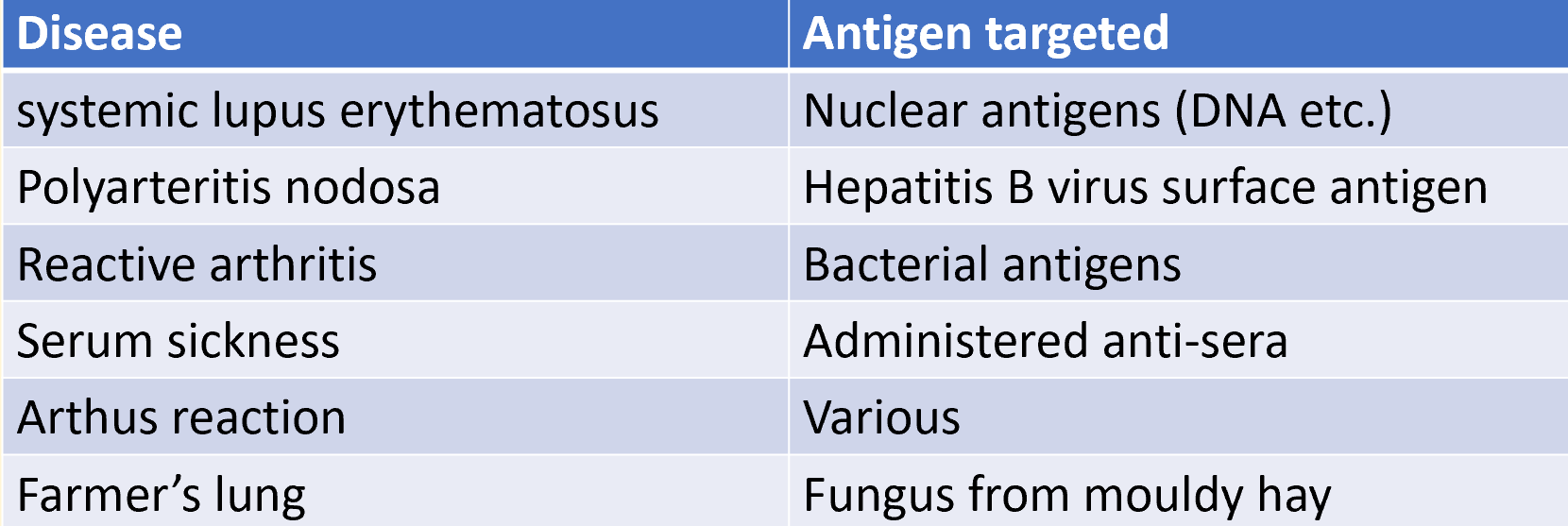
type III hypersensitivity is mediated by antigen-AB complexes
large quantities of self or non self antigen reach the blood and form complexes with specific IgG or IgM ABs
such circulating immune complexes are commonly generated at a low level during infections and part of normal body processes
these immune complexes are cleared quickly from the circulation by the liver/spleen with help of complement binding
systemic lupus erytheamatosus
autoimmune disease driven by immune complexes formed between autoABs and host cell nuclear antigens
common symptoms: rashes, arthritis, glomerulonephritis
has both genetic and environmental risk factors
affects women more commonly than men
serum sickness
caused by injection of large quantities of a poorly catabolised foreign antigen
occurs 7-10 days after injection
ABs form immune complexes with their antigens, activate complement and bind Fc receptors on leukocytes (tissue damage)
symptoms:
urticaria (rash): histamine from mast cell degranulation (via FcγRIII by IgG immune complexes and anaphylatoxins C3a and C5a released due to complement activation)
injury to tissues/organs (skin, kidneys, nerves)
diseases caused by type IV hypersensitivity
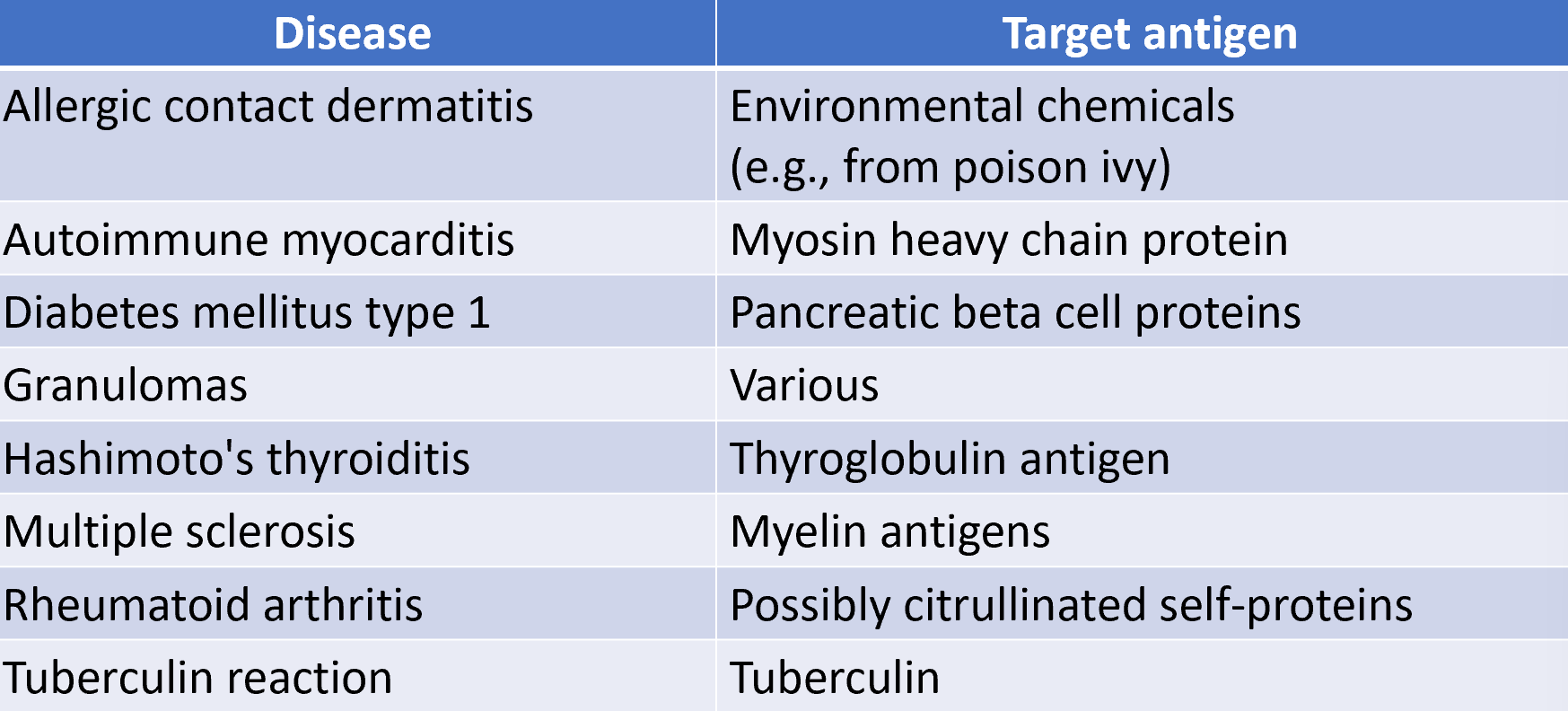
type IV hypersensitivity mediated by CD4+ or CD8+ T cells (delayed type hypersensitivity)
often caused by cytokines from activated CD4+ Th cells, particularly Th1 and Th17 cells
CD8+ T cell responses may also promote a type IV hypersensitivity response
responses are directed against a protein or peptide epitope to which effector T cells have already been generated
CD4+ T cell responses are believed to play a key role in many autoimmune diseases
often a long lag phase between exposure to the antigen and the resulting inflammatory response
delayed type hypersensitivity can fall under 3 major categories
contact
T cell responses to environmental antigens from skin contact
2-3 days max reaction time
granulomatous
T cell cytokines promote formation of giant cells from macrophages
2-3 weeks
tuberculin
T cell response to injected tuberculin
2-3 days
type V hypersensitivity: stimulatory reactions
ABs activate receptors and unwanted outcomes
grave’s disease
myasthenia gravis
Grave’s Disease
Grave’s hyperthyroidism is caused by the stimulation of thyroid stimulating hormone (TSH) receptor by TSH receptor autoABs
approx 35-50% of patients with Grave’s have clinical eye involvement
most Grave’s opthalmology patients have an increase in both orbital fat and extraocular muscle volumes
myasthenia gravis
long term neuromuscular disease that leads to varying degrees of skeletal muscle weakness
commonly affected muscles: eyes, face and swallowing
ABs inhibit binding of Ach to Ach receptor, inhibiting transmission of signals from nerve endings to muscle fibres