resting membrane potential
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
general concepts
Lab goal: examine how resting membrane potential (RMP) depends on extracellular K⁺ concentration.
In the lab, you will measure resting membrane potential using electrodes.
RMP definition: inside of cell is negative relative to the outside (extracellular = 0 mV by convention).
Typical RMP values: −70 to −90 mV.
how to estimate number of charges involves
To estimate charge separation:
Calculate cell surface area (from radius & shape).
Membrane capacitance per unit area is ~ 1 μF/cm² (constant across cells).
Total membrane capacitance:
C_total = (capacitance/unit area) × (surface area).
Use Coulomb’s law:
Q = C × U, where
Q = charge,
C = capacitance,
U = membrane potential.Elementary charge = 1.6 × 10⁻¹⁹ C → allows conversion of Q into number of ions.
Also possible to estimate total ionic charge in a cell:
Intracellular ion concentration ~ 140 mM (for many ions).
Knowing cell volume allows calculation of total # ions.
Comparison:
Only a tiny fraction of total ions participate in the membrane charge separation → important conceptual implication.
Both intra- and extracellular solutions are electrically neutral overall, but at the membrane a very thin layer of charge is separated:
Inside surface = negative
Outside surface = positive
what determines rmp
Ion concentration gradients across the membrane
(especially Na⁺, K⁺, Cl⁻)Relative membrane permeabilities to those ions.
Major ions considered: Na⁺, K⁺, Cl⁻ (others exist but less influential).
ionic asymmetry
Intracellular concentrations:
K⁺ high, Na⁺ low.
Extracellular concentrations:
K⁺ low, Na⁺ high.
This asymmetry is established and maintained by:
Na⁺/K⁺ ATPase pump:
Pumps 3 Na⁺ out
Pumps 2 K⁺ in
Keeps intracellular K⁺ high, intracellular Na⁺ low.
how does ionic asymmetry + selective permeability = membrane potential
With an ion gradient + membrane permeable only to one ion, a membrane potential must develop.
Simplified 2-compartment model:
Both compartments start electrically neutral, same ions (K⁺, Cl⁻).
Membrane permeable only to K⁺.
One side has high K⁺, the other low K⁺.
Chemical force (concentration gradient) pushes K⁺ from high → low concentration side.
Receiving compartment becomes progressively more positive (K⁺ enters).
Donor compartment becomes progressively more negative (extra Cl⁻ left behind).
This creates an electrical force:
Positive compartment repels further K⁺ entry.
Negative compartment attracts K⁺ back.
Electrical force increases as charge separation grows.
At equilibrium:
Chemical force = electrical force (equal & opposite).
No net K⁺ movement.
The voltage at this point = equilibrium potential (E_K).
Nernst equation
Physical chemist Nernst derived the equation relating equilibrium potential to ion concentration ratio.
Full form includes:
R (gas constant), T (Kelvin temperature), z (ion valence), F (Faraday constant).At room temperature, simplified:
E = 58 mV × log₁₀([ion]ₒᵤₜ / [ion]ᵢₙ)Key points:
Depends only on ratio of concentrations.
Does not depend on permeability, as long as permeability is nonzero.
Each 10-fold change in [ion]ₒᵤₜ changes E by 58 mV
hypothesis in lab
Hypothesis: at rest, membrane is permeable only to K⁺.
If true:
Plotting RMP vs. log([K⁺]ₒᵤₜ) should produce a straight line (same slope as Nernst prediction: 58 mV/decade).
Experimentally:
Plot shows a line, and experimental RMP values (circles) closely follow predicted E_K.
Not perfect match at low K⁺ concentrations.
Why imperfect agreement?
Membrane is not exclusively permeable to K⁺.
There is some Na⁺ permeability.
E_Na ≈ +50 to +60 mV (positive because [Na⁺]ₒᵤₜ is high, [Na⁺]ᵢₙ is low).
Mixed Na⁺ permeability pulls RMP slightly more positive than pure E_K prediction.
effects of changing extracellular K+
If [K⁺]ₒᵤₜ increases 10×, E_K becomes 58 mV more positive.
If [K⁺]ₒᵤₜ increases 100×, E_K becomes 116 mV more positive (because 58 × 2).
Result of the logarithmic relationship (semi-log plot).
recording setup
muscle fibre (cylindrical), recording instruments.
Two electrodes:
Bath electrode in the extracellular solution.
Glass microelectrode inserted into muscle fibre.
Glass microelectrode
Glass, tapered tip.
Filled with electrolyte.
Inserted into the muscle cell to record electrical potential
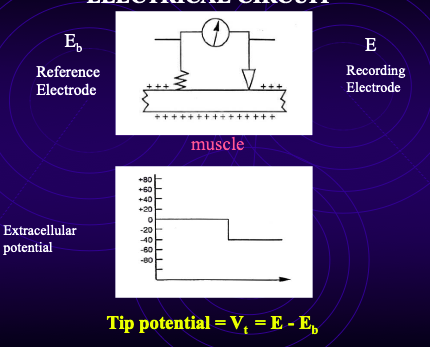
tip potential
When the microelectrode is placed in the bath, the potential ≠ 0.
You measure a tip potential (VT):
Caused by charges at the tip altering ionic environment inside vs outside electrode.
recording electrode - reference electrode
Importance:
When electrode enters the cell, measured potential (VX) = RMP + tip potential.
Must subtract tip potential to obtain true RMP.
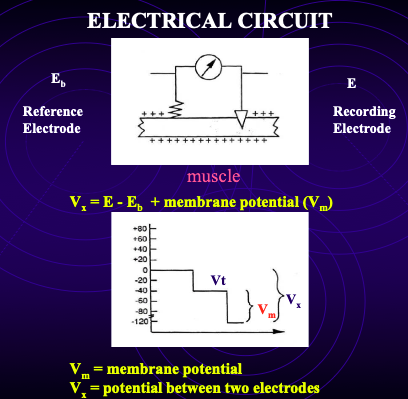
how to measure voltage
Place electrode in bath → record VT (tip potential).
Insert electrode into muscle fibre → measure VX.
RMP = VX – VT (aka potential between the two electrodes - (recording electrode - reference electrode))
electrode design requirements
Tip size
Must be very small (~ 1 μm diameter).
Prevents physical damage to muscle fibre.
Large damage → intracellular fluid leaks out, extracellular fluid leaks in → disrupts measurements.
Tip size affects resistance
Normal resistance for 1 μm tip ≈ 10 MΩ.
If tip breaks → resistance decreases.
If tip becomes plugged (e.g., connective tissue) → resistance increases
internal electrode solution
Filled with 3 M KCl (very high concentration).
High concentration needed to allow strong ionic current → good electrical contact.
Why not NaCl?
If electrode leaks:
Na⁺ leak would dramatically alter intracellular Na⁺ concentration.
K⁺ leak is safer because cell already has high intracellular K⁺.
Comparisons:
Intracellular K⁺ ≈ 140 mM.
Electrode KCl = 3 M (much higher).
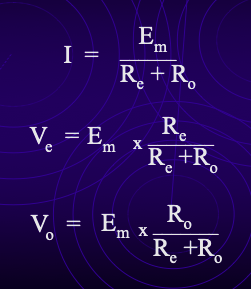
recording instrument requirements
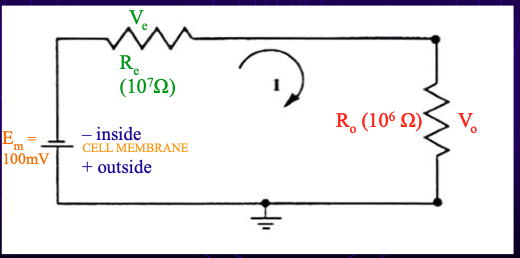
Equivalent circuit sketch
Em = true resting membrane potential (modelled as a battery).
Re = electrode resistance (≈10 MΩ).
Ro = input resistance of recording instrument.
problem w oscilloscope
Oscilloscope input resistance ≈ 1 MΩ.
Measurement:
Voltage measured = voltage drop across RO.
Most voltage drops across RE instead → huge signal loss.
Calculation:
RO = 1 MΩ, RE = 10 MΩ → total = 11 MΩ.
Only ~1/11 (~9%) of true RMP is recorded.
Conclusion: oscilloscope alone is not suitable.
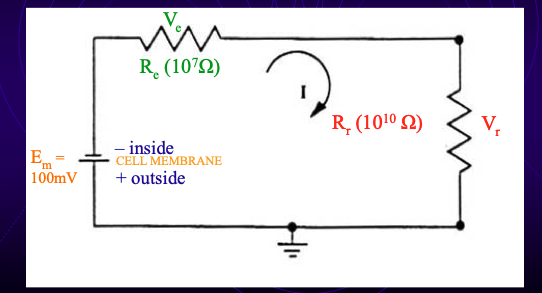
solution to problem with oscilloscope
Use amplifier where RO is very large (≥ 10¹⁰ Ω; in reality ~10¹⁴ Ω).
Then:
RO >> RE → nearly all voltage drop across RO.
Ratio RO / (RE + RO) ≈ 1.
Measured voltage ≈ true RMP.
why is high input resistance necessary
Accurate RMP measurement
Minimizes voltage loss across electrode.
2. Reduces current flow through electrode
High current → lots of ion movement → disturbs intracellular environment.
Want minimal disturbance.
3. Electrode resistance often changes
When entering/leaving the cell, brushing tissue, plugging, etc.
High RO ensures changes in RE do not affect recorded RMP.
Because RO >> RE, even if RE changes from 10 → 1 or 20 MΩ, proportion stays ~1.
potassium vs sodium equilibrium potentials
EK ≈ –90 mV (example: –90 to –110 mV range).
ENa ≈ +60 mV.
If membrane were equally permeable to K⁺ and Na⁺
RMP would be halfway between:
(–90 + +60) / 2 = –15 mV.
If membrane permeable only to K⁺
RMP = EK ≈ –90 mV.
If membrane permeable only to Na⁺
RMP = ENa ≈ +60 mV.
In reality
Membrane is much more permeable to K⁺, so RMP is close to EK.
experimental procedure
Record RMP at low extracellular K⁺.
Increase extracellular K⁺ stepwise (5 different concentrations).
Measure RMP at each condition.
Graph the relationship:
Does RMP follow predicted Nernst-like slope?
Perform statistical tests:
Compare low vs high K⁺.
Use t-tests, p-values.
TEA experiments
TEA blocks some K⁺ channels → reduces K⁺ conductance.
What should happen if K⁺ is fully blocked?
RMP ≈ ENa (≈ +60 mV), and changes in external K⁺ would have no effect.
But TEA does not block all K⁺ channels:
Many potassium channel subtypes (different conductance, pharmacology, open times).
TEA blocks only some of them → partial conductance remains.
What you will observe
With TEA:
K⁺ conductance decreases → RMP becomes less dependent on extracellular K⁺.
RMP shifts toward more positive values (toward ENa).
But does not reach +60 mV because some K⁺ channels still function.