Year 9 Chemistry, Chemistry Revision Year 9, Year 9 Chemistry
5.0(1)
Card Sorting
1/94
Last updated 9:25 AM on 11/15/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
95 Terms
1
New cards
A class of elements characterized by physical properties that include shininess, malleability, ductility, and conductivity.
Metal

2
New cards
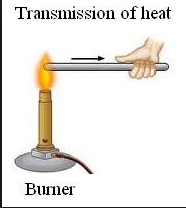
The ability of an object to transfer heat or electricity to another object.
Conductivity
3
New cards
Able to be stretched into wires
Ductile

4
New cards
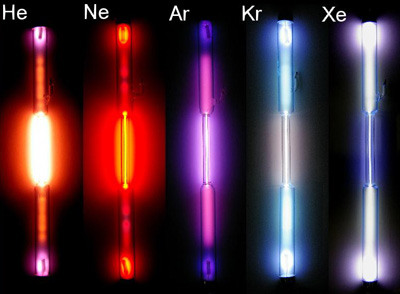
Elements in Group 18 of the periodic table. Have no charge and are gases under normal conditions. (Helium, Neon, Argon, Krypton, Xenon, Radon)
Noble gases
5
New cards
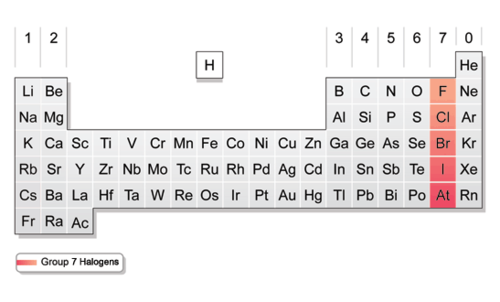
Contains nonmetals, 7 valence electrons in it's outermost energy level. Very reactive
Halogens
6
New cards
Group 2 elements
Alkali Earths
7
New cards
The attraction between oppositely charged ions
Ionic bonding
8
New cards
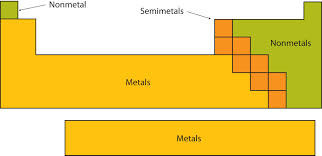
An element that tends to be a poor conductor of heat and electric current; these generally have properties opposite to those of metals.
Non-metals
9
New cards
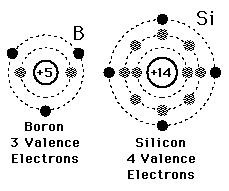
Electrons on the outermost energy level of an atom
Valence electrons
10
New cards
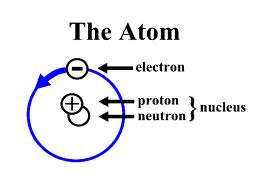
The basic building block of all materials; it consists of neutrons and protons surrounded by a cloud of electrons
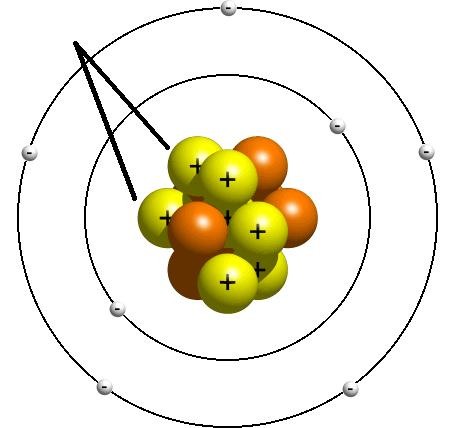
Atom
11
New cards
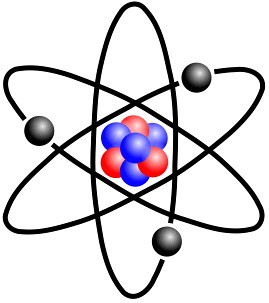
The fundamental building block of all materials; it consists of a bunch of neutrons and protons surrounded by a cloud of electrons
Atomic model
12
New cards
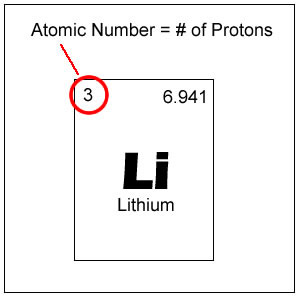
The number of protons in a neucleus; the atomic number determines what type of atom it is
Atomic number
13
New cards
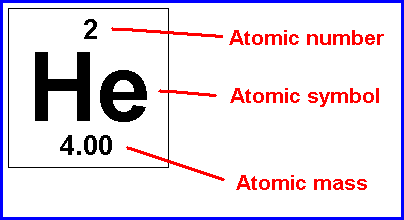
A short-hand notation for describing an atom; it consists of the chemical "letter(s)", atomic number and mass number
Atomic symbol
14
New cards
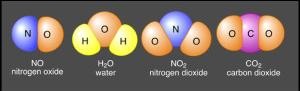
A pure substance that is made up of two or more different types of atom chemically bound together
Compound
15
New cards
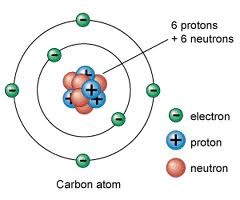
A small, negatively charged particle; clouds of electrons surround the nucleus of an atom
Electron
16
New cards
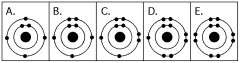
The number of electrons in each of the electron shells of an atom
Electron configuration
17
New cards
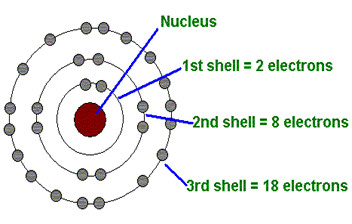
Part of the electron cloud; it is a layer that surrounds the nucleus and can only hold a certain number of electrons
Electron shell
18
New cards
A substance made up of only one type of atom
Element
19
New cards
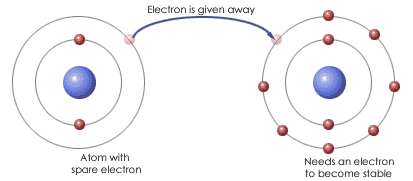
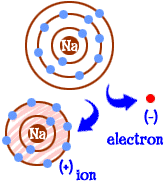
An atom that has gained or lost electrons and therefore has an electric charge
Ion
20
New cards
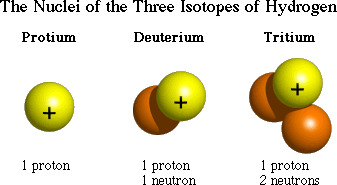
Atoms that have the same number of protons (atomic number) but a different number of neutrons in their nucleus
Isotope
21
New cards
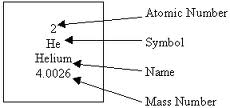
The number of protons and neutrons in an atom's neucleus
Mass number
22
New cards
A cluster of atoms that makes up an element or a compound
Molecule
23
New cards
Nuclear radiation
Describes any rays or particles released by atomic nuclei
24
New cards
Nucleus
The centre of an atom, containing protons & neutrons
25
New cards
Chemical properties
Characteristics of a substance that determine how it will react with other substances.

26
New cards
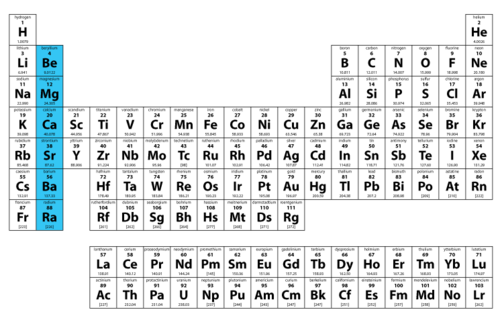
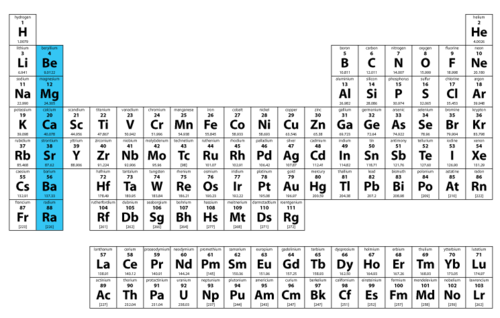
Periodic table
A table showing the symbols and often data of all 118 known types of atoms (elements)
27
New cards
Proton
A positively charged particle in the nucleus
28
New cards
3
Atomic Number of Lithium
29
New cards
Li
Symbol for Lithium
30
New cards
Group 1
Lithium's Group.
31
New cards
Group 14
Silicon's Group.
32
New cards
Group 16
Sulfur's Group
33
New cards
Group 2
Calcium's Group
34
New cards
16
Atomic Number of Sulfur
35
New cards
20
Atomic Number of Calcium
36
New cards
Period 3
Silicon's Period
37
New cards
Period 2
Lithium's Period
38
New cards
Period 4
Calcium's Period
39
New cards
0
Neutrons in Hydrogen
40
New cards
10
Neutrons in Fluorine
41
New cards
14
Neutrons in Aluminium
42
New cards
9
Fluorine's Electrons
43
New cards
15
Atomic Number of Phosphorus
44
New cards
27
Mass Number for Aluminium
45
New cards
19
Mass number for Fluorine
46
New cards
2,6
Electron configuration for Oxygen
47
New cards
2,4
Electron configuration for Carbon
48
New cards
2,8,8
Electron configuration for Argon
49
New cards
2,8,8,2
Electron configuration for Calcium
50
New cards
+
Charge on a proton
51
New cards
-
Charge on an electron
52
New cards
No charge
Charge of a neutron
53
New cards
Proton and Electron
Equal but opposite charges
54
New cards
Proton and Neutron
Both live in the nucleus
55
New cards
1 atomic mass unit
The weight of protons or neutrons.
56
New cards
1/1800th of an atomic mass unit.
The weight of electrons.
57
New cards
Mass number
protons + neutrons
58
New cards
In a normal atom:
Number of electrons =
Number of electrons =
Number of protons
59
New cards
In an ion:
Number of electrons =
Number of electrons =
Changes (more or less) to get a full outer shell
60
New cards
Atomic substance
Atoms that are not bonded to other atoms. e.g. noble gases
61
New cards
Molecular substance
Contains molecules, each molecules has two or more atoms bonded together.
62
New cards
Lattice
Interconnected atoms or ions, all bonded together to form a pattern.
63
New cards
Isotopes will have the same number of
Protons
64
New cards
Isotopes will have different numbers of
Neutrons
65
New cards
Metal
A class of elements characterized by physical properties that include shininess, malleability, ductility, and conductivity.

66
New cards
Conductivity
The ability of an object to transfer heat or electricity to another object.
67
New cards
Malleable
A class of elements characterized by physical properties that include shininess, malleability, ductility, and conductivity

68
New cards
Ductile
Able to be stretched into wires

69
New cards
Reactivity series
A list of metals which shows them in order of their reactivity, with the most reactive at the top.
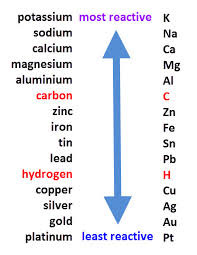
70
New cards
Electronegativity
A measure of the ability of an atom in a chemical compound to attract electrons
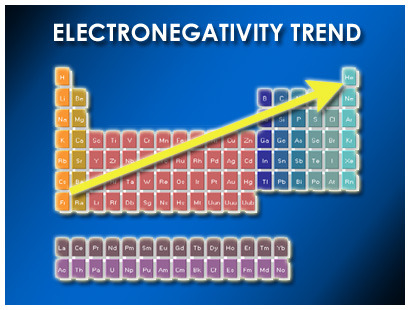
71
New cards
Noble gases
Elements in Group 18 of the periodic table. Have no charge and are gases under normal conditions. (Helium, Neon, Argon, Krypton, Xenon, Radon)
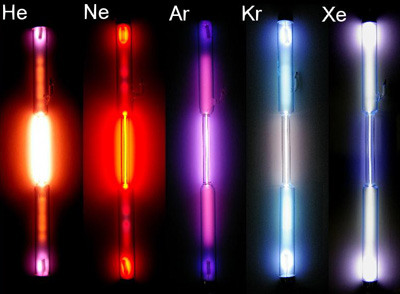
72
New cards
Halogens
Contains nonmetals, 7 valence electrons in it's outermost energy level. Very reactive
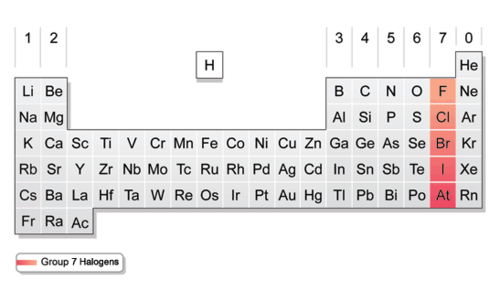
73
New cards
Alkali Earths
Group 2 elements
74
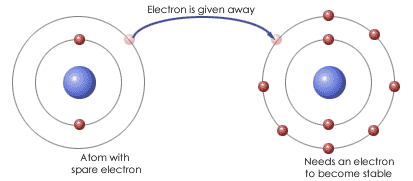
New cards
Ionic bonding
The attraction between oppositely charged ions
75
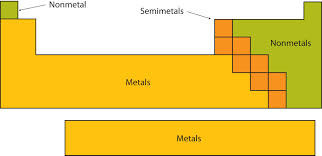
New cards
Non-metals
An element that tends to be a poor conductor of heat and electric current; these generally have properties opposite to those of metals.
76
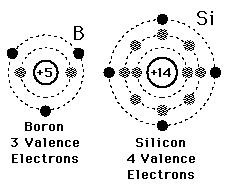
New cards
Valence electrons
Electrons on the outermost energy level of an atom
77
New cards
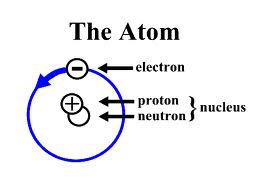
Atom
The basic building block of all materials; it consists of neutrons and protons surrounded by a cloud of electrons
78
New cards
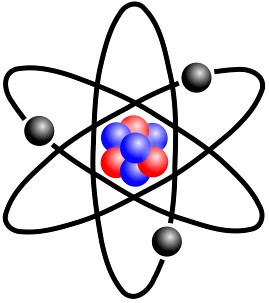
Atomic model
The fundamental building block of all materials; it consists of a bunch of neutrons and protons surrounded by a cloud of electrons
79
New cards
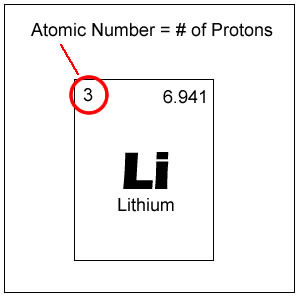
Atomic number
The number of protons in a neucleus; the atomic number determines what type of atom it is
80
New cards
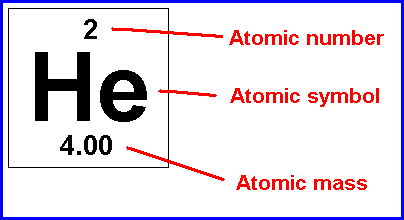
Atomic symbol
A short-hand notation for describing an atom; it consists of the chemical "letter(s)", atomic number and mass number
81
New cards
Compound
A pure substance that is made up of two or more different types of atom chemically bound together
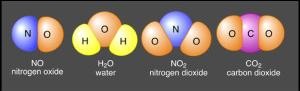
82
New cards
Electron
A small, negatively charged particle; clouds of electrons surround the nucleus of an atom
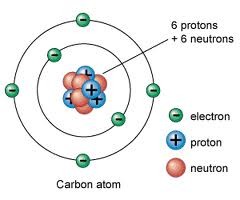
83
New cards
Electron configuration
The number of electrons in each of the electron shells of an atom
84
New cards
Electron shell
Part of the electron cloud; it is a layer that surrounds the nucleus and can only hold a certain number of electrons
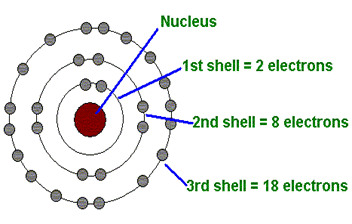
85
New cards
Element
A substance made up of only one type of atom
86
New cards
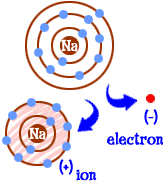
Ion
An atom that has gained or lost electrons and therefore has an electric charge
87
New cards
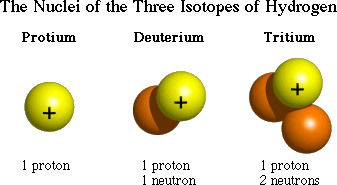
Isotope
Atoms that have the same number of protons (atomic number) but a different number of neutrons in their nucleus
88
New cards
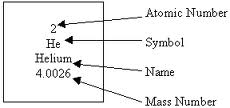
The number of protons and neutrons in an atom's nucleus
Mass number
89
New cards
Molecule
A cluster of atoms that makes up an element or a compound
90
New cards
Neutron
A particle in the nucleus of an atom having the same mass as a proton but no overall charge
91
New cards
Describes any rays or particles released by atomic nuclei
Nuclear radiation
92
New cards
The centre of an atom, containing protons & neutrons
Nucleus
93
New cards
Characteristics of a substance that determine how it will react with other substances.
Chemical properties

94
New cards
A table showing the symbols and often data of all 118 known types of atoms (elements)
Periodic table
95
New cards
A positively charged particle in the nucleus
Proton