PERIODICITY
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
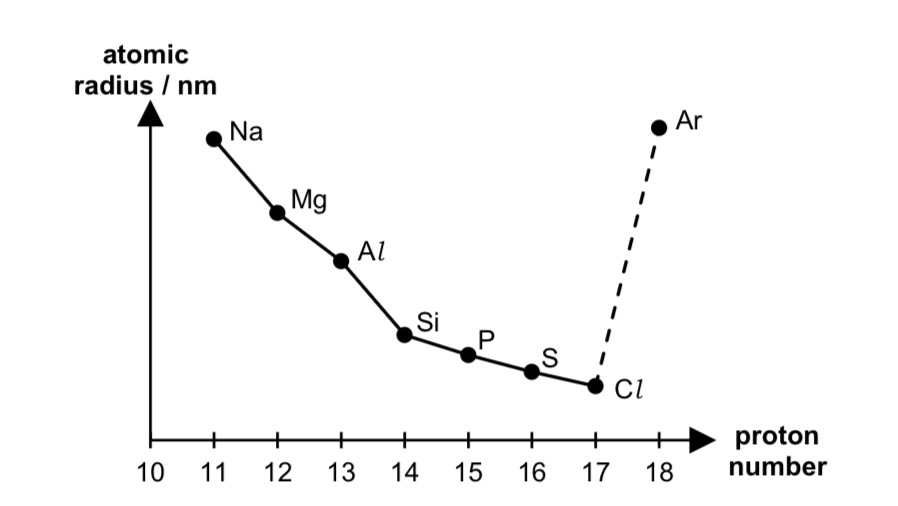
describe the trend in atomic radii across period 3.
across the period, atomic radius decreases because
no. of protons in nucleus increases, thus nuclear charge increases.
there is the same no. of inner shell electrons, thus shielding effect is relatively constant.
hence increase in effective nuclear charge across period,
and increase in electrostatic forces of attraction between nucleus and valence electrons
exception of Ar: largest as it has van der Waals’ radius
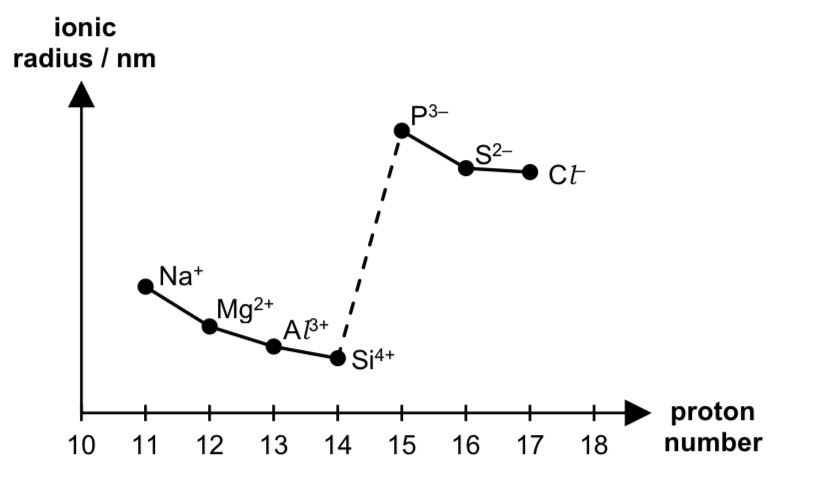
describe the trend in ionic radii across period 3
cationic radius is smaller than anionic radius as anions have one more filled principle quantum shell compared to cations.
valence electrons in cations are closer to the nucleus than those in anions,
leading to strong electrostatic forces of attraction between nucleus and valence electrons of cations than anions.
in cations, from Na+ to Si4+, ionic radii decreases because NC increases.
the cations have same inter-electronic repulsion since cations have same no. of electrons,
hence there are stronger electrostatic foa between nucleus and valence electrons,
leading to cationic radius to decrease across the period.
in anions, from P3- to Cl-, ionic radii decreases because NC increases.
there is same inter-electronic repulsion since anions have same no. of electrons,
hence there are stronger electrostatic foa between nucleus and valence electrons,
leading to anionic radius to decrease across the period.
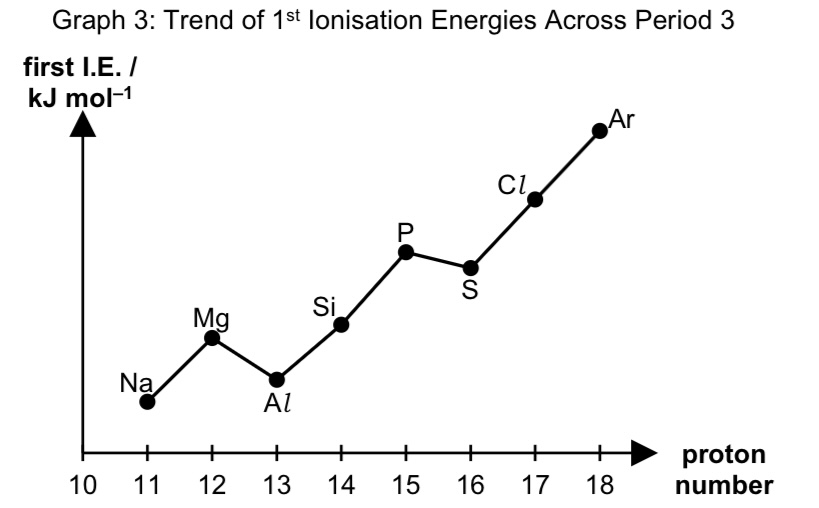
describe the trend in 1st ionisation energy across period 3
across period 3, 1st IE generally increases because
no. protons in nucleus of atom increases, thus NC increases.
there are same no. of inner shell electrons, thus SE is relatively constant, thus there is an increase in effective nuclear charge,
and increase in electrostatic foa between the nucleus and valence electron,
hence more energy is needed to remove the valence electron.
exceptions:
1st IE of Al is lower than Mg as
the 3p electron to be removed in Al is at higher energy level than the 3s electron to be removed from Mg,
hence less energy is required to remove the 3p electron from Al.
1st IE of S is lower than P because
there is inter-electronic repulsion between the paired electrons in the same orbital for S,
thus less energy is required to remove a paired 3p electron from S as compared to an unpaired 3p electron removed in P.
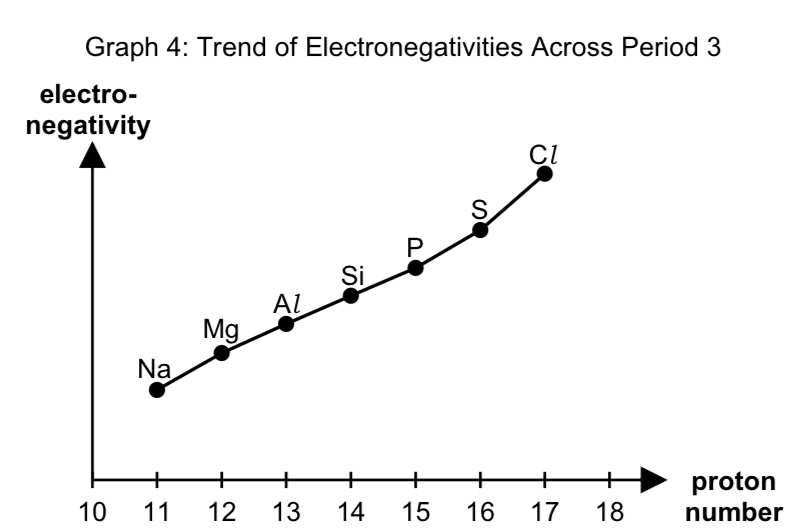
describe the trend of electronegativity across period 3
across period 3, electronegativity increases as
no. of protons in nucleus of atom increases, thus NC increases.
there is the same no. of inner shell electrons thus SE is relatively constant constant, leading to increase in ENC.
hence increase in electrostatic foa between the nucleus and valence electron
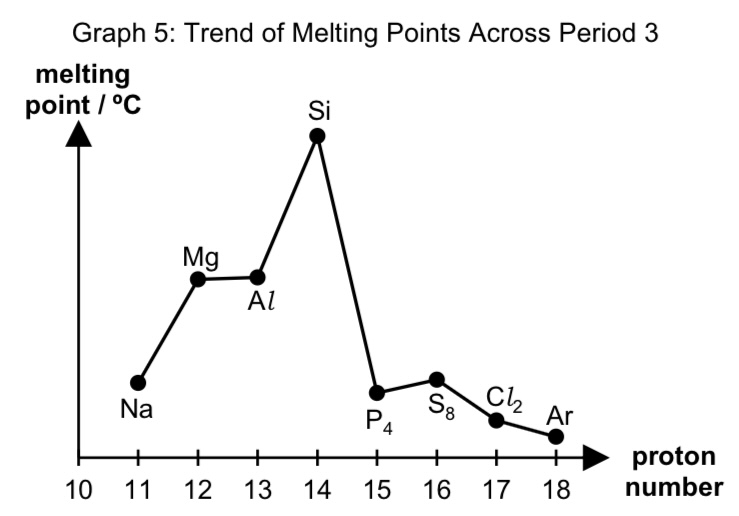
describe the trend of melting points across period 3
from Na to Al, melting points are high and increase due to increasing metallic bond strength as no. of delocalised electrons increases.
since NC increases and cationic radii decreases from Na to Al, charge density increases, and
more energy is needed to overcome the strong electrostatic foa between metal cations and sea of delocalised electrons.
Si has the highest melting point due to its giant molecular structure which
requires a large amount of energy to overcome the strong extensive covalent bonds between atoms.
from P to Ar, the melting points are low because of their simple molecular structure
which only requires a small amount of energy to overcome the weak intermolecular instantaneous dipole-induced dipole interactions.
melting points of S8 > P4 > Cl2 > Ar because of the increase in no. of electrons in the molecule,
leading to increase in polarisability of electron cloud,
hence more energy is required to overcome stronger intermolecular id-id interactions.
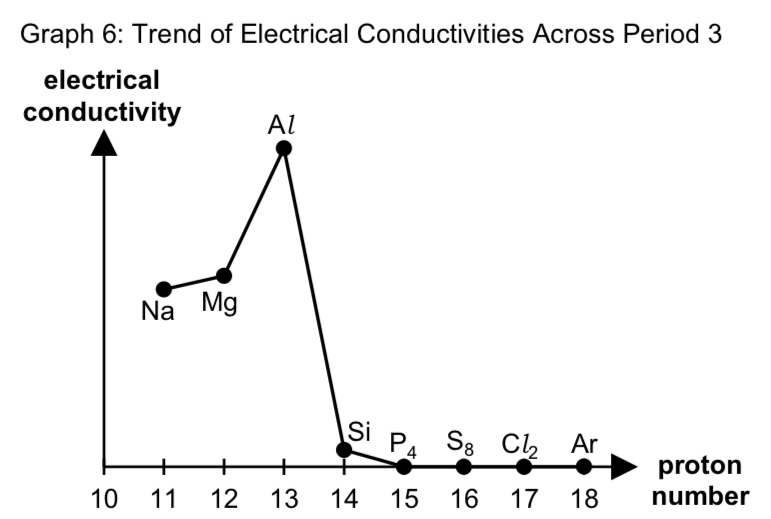
describe the trend of electrical conductivity across period 3
from Na to Al, there is high electrical conductivity which increases due to increase in no. of delocalised electrons
which act as mobile charge carriers to conduct electricity.
compared to the period 3 metals, Si has a low electrical conductivity as it is a metalloid
while P, S, Cl and Ar are non-conductors of electricity in any state due to electrons being localised in covalent bonds
hence there is an absence of delocalised electrons or mobile ions.
sketch the graph to show the variation in atomic radii across period 3
sketch the graph to show the variation in ionic radii across period 3
sketch the graph to show the variation in 1st ionisation energies across period 3
sketch the graph to show the variation in electronegativity across period 3
sketch the graph to show the variation in melting points across period 3
sketch the graph to show the variation in electrical conductivity across period 3
which of the elements in period 3 react with water/steam?
Na and Mg.
What are the equations for the reactions of Na and Mg with water?
Na + H2O → NaOH + ½ H2
Mg + H2O → MgO + H2
Why can P and S exhibit multiple oxidation numbers?
P, +3 and +5
S, +4 and +6
presence of vacant and energetically accessible d orbitals which can be used for bonding through expansion of octet structure
describe the trend of melting points of period 3 oxides till SO3
from Na2O to Al2O3, the melting points are high due to large amount of energy needed to overcome the strong ionic bonds.
the mp of MgO is higher than Na2O because although anionic charge and anionic radius remains the same,
Mg2+ has a higher ionic charge and smaller ionic radius than Na+, hence magnitude of lattice energy of MgO is greater than that of Na2O
the mp of MgO is higher than Al2O3 because
Al3+ has higher ionic charge and smaller ionic radius than Mg2+,
Al3+ has higher charge density and hence higher polarising power,
thus Al2O3 has some covalent character, weakening the ionic bonds in Al2O3
SiO2 has a high mp because a large amount of energy is required to overcome the strong extensive covalent bonds between the atoms
P4O10 and SO3 have simple molecular structure which have low mp because a small amounts of energy is needed to overcome the weak intermolecular id-id interactions
mp of P4O10 is higher than that of So3 because there are more electrons in P4O10 molecule than SO3,
so there is an increase in polarisability of electron cloud for P4O10,
and more energy is required to overcome the stronger intermolecular id-id interactions for P4O10
describe the trend of melting points of period 3 chlorides, till PCl5
NaCl and MgCl2 have high mp due to large amount of energy needed to overcome the strong ionic bonds
NaCl has a higher mp than MgCl2 because Mg2+ has higher ionic charge and smaller ionic radius than Na+,
thus Mg2+ has higher charge density and hence higher polarising power than Na+,
so MgCl2 has some covalent character, weakening the ionic bonds in MgCl2.
AlCl3, SiCl4, PCl5 have low mp due to small amount of energy needed to overcome the weak intermolecular id-id interactions
PCl5 has higher mp than SiCl4, because there are more electrons in PCl5 molecule than SiCl4,
increasing the polarisability of the electron cloud for PCl5,
hence more energy is required to overcome the stronger intermolecular id-id interactions for PCl5
describe the structures of period 3 oxides
Na2O, MgO, Al2O3 - giant ionic crystal lattice structure
SiO2 - giant molecular structure
P4O10, SO3 - simple molecular structure
describe the structures of period 3 chlorides
NaCl, MgCl2 - giant ionic crystal lattice structure
AlCl3, SiCl4, PCl5 - simple molecular structure
why does AlCl3 have simple molecular structure?
Al3+ has high ionic charge and small ionic radius,
hence Al3+ has higher charge density and hence high polarising power
Al-Cl bond has significant covalent character, hence AlCl3 has simple molecular structure
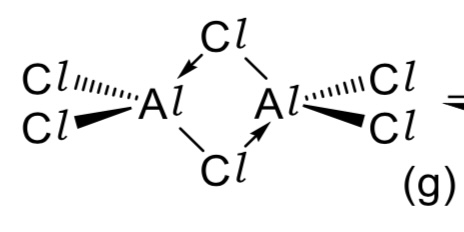
draw the structure of Al2Cl6
describe the reactions of period 3 oxides with water, their equations, and the pH of the resulting solution.
Na2O dissolves completely in water to form NaOH.
Na2O (s) + H2O (l) → 2NaOH (aq)
pH 14
MgO dissolves partially in water to form Mg(OH)2.
MgO (s) + H2O (l) → Mg(OH)2 (s)
pH 9
Al2O3 does not dissolve in water due to its extremely high LE. Large amt energy needed to break strong ionic bonds.
pH 7
SiO2 does not dissolve in water because large amt energy required to break strong and extensive covalent bonds between Si and O atoms.
pH 7
P4O10 dissolves in water to form H3PO4.
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
pH 2
SO3 dissolves in water to form H2SO4.
SO3 (g) + H2O (l) → H2SO4 (aq)
pH 2
describe the reactions of period 3 oxides with acids and bases.
REACTION WITH ACID
Na2O forms salt and water. Na2O (s) + 2HCl (aq) → 2NaCl (aq) + H2O (l)
MgO forms salt and water. MgO (s) + 2HCl (aq) → MgCl2 (aq) + H2O (l)
Al2O3 forms salt and water. Al2O3 (s) + 6HCl (aq) → 2AlCl3 (aq) + 3H2O (l)
SiO2, P4O10, and SO3 have no reaction.
REACTION WITH BASE
Na2O, MgO have no reaction.
Al2O3 dissolves in excess NaOH to form colourless complex [Al(OH)4]- (aq). Al2O3 (s) + 2NaOH (aq) + 3H2O (l) → NaAl(OH)4 (aq)
SiO2 reacts with hot conc NaOH to form Na2SiO3 (aq). SiO2 (s) + 2NaOH (conc) → Na2SiO3 (aq) + H2O (l)
P4O10 reacts to form salt and water. P4O10 (s) + 12NaOH (aq) → 4Na3PO4 (aq) + 6H2O (l)
SO3 reacts to form salt and water. SO3 (g) + 2NaOH (aq) → Na2SO4 (aq) + H2O (l)
describe the natures of the period 3 oxides
Na2O and MgO are basic.
Al2O3 is amphoteric.
SiO2, P4O10, SO3 are acidic.
describe the structure and nature of period 3 hydroxides
NaOH, Mg(OH)2, Al(OH)3 have giant ionic structure
NaOH and Mg(OH)2 are basic while Al(OH)3 is amphoteric
describe the reactions of period 3 hydroxides with acids and bases
REACTION WITH ACID
NaOH forms salt and water. NaOH (aq) + H+ (aq) → Na+ (aq) + H2O (l)
Mg(OH)2 forms salt and water. Mg(OH)2 (s) + 2H+ (aq) → Mg2+ (aq) + 2H2O (l)
Al(OH)3 forms salt and water. Al(OH)3 (s) + 3H+ (aq) → Al3+ (aq) + 3H2O (l)
REACTION WITH BASE
NaOH and Mg(OH)2 do not react.
Al(OH)3 dissolves in excess NaOH to form colourless complex, [Al(OH)4]- (aq)
Al(OH)3 (s) + OH- (aq) → [Al(OH)4]- (aq)
which period 3 chlorides undergo hydration and/or hydrolysis?
HYDRATION
NaCl, MgCl2, AlCl3
undergo hydration to give hydrated metal ions and chloride ions. (formation of ion-dipole interactions)
SiCl4, PCl5 DO NOT UNDERGO HYDRATION
HYDROLYSIS
[Na(H2O)6]+ DOES NOT UNDERGO HYDROLYSIS
[Mg(H2O)6]+ undergoes slight hydrolysis as Mg2+ has higher charge density than Na+, which can polarise the electron cloud of surrounding water ligands, weakening and breaking O-H bond, resulting in release of H+.
[Al(H2O)6]+ undergoes extensive hydrolysis, more extensive than that of [Mg(H2O)6]+ as Al3+ has higher charge density than Mg2+
AlCl3, SiCl4, PCl5 undergo complete hydrolysis. Si and P atoms have vacant and energetically accessible 3d orbitals. Al atom has vacant and energetically accessible 3p orbital to accept lone pair from H2O molecules, forming dative bond.
describe the reactions of period 3 chlorides with water, their reactions and the pH of the resulting solution
NaCl dissolves completely in water.
NaCl (s) + aq → Na+ (aq) + Cl- (aq) (hydration)
pH 7
MgCl2 dissolves completely in water.
MgCl2 (s) + 6H2O (l) → [Mg(H2O)6]2+ (aq) + 2Cl- (aq) (hydration)
[Mg(H2O)6]2+ (aq) ⇌ [Mg(H2O)5(OH)]+ (aq) + H+ (aq) (slight hydrolysis)
pH 6.5
AlCl3 dissolves completely in excess water.
AlCl3 (s) + 6H2O (l) → [Al(H2O)6]3+ (aq) + 3Cl- (aq) (hydration)
[Al(H2O)6]3+(aq) ⇌ [Al(H2O)5(OH)]2+ (aq) + H+ (aq) (extensive hydrolysis)
pH 3
In limited amount of water, AlCl3 reacts vigorously with water, forming a white solid and white fumes of HCl.
AlCl3 (s) + 3H2O (l) → Al(OH)3 (s) + 3HCl (g) (complete hydrolysis) /
2AlCl3 (s) + 3H2O (l) → Al2O3 (s) + 6HCl (g)
pH 3
SiCl4 reacts vigorously with water, and forms white solid with white fumes of HCl.
SiCl4 (l) + 2H2O (l) → SiO2 (s) + 4HCl (aq) (complete hydrolysis)
pH 2
PCl5 reacts vigorously with water, forms white fumes of HCl.
PCl5 (s) + 4H2O (l) → H3PO4 (aq) + 5HCl (aq) (complete hydrolysis)
pH 2
Why do diagonal relationships occur between period 2 and 3?
they occur because
the elements have similar electronegativity (increases across period and decreases down the group)
their cations have similar charge density (charge density increases across the period and radius increases down the group)
electronic configuration, atomic and ionic radius, first IE, and electronegativity are determined by
atomic structure
melting point is determined by
chem bonding
describe the trend of atomic and ionic radius for group 2 elements.
down the group,
both NC and SE increase,
however, valence electrons are located in a shell with larger principle quantum no. , and are further away from the nucleus
weaker electrostatic foa between nucleus and valence electrons lead to atomic and ionic radius increasing down the group
describe the trend of first IE down group 2.
down the group,
both NC and SE increase,
however, valence electrons are located in a shell with larger principle quantum no. , and are further away from the nucleus.
hence there are weaker electrostatic foa between the nucleus and valence electrons,
and less energy is needed to remove the valence electron
thus first IE decreases
describe the trend of electronegativity down group 2
down the group,
both NC and SE increase,
however, valence electrons are located in a shell with larger principle quantum no. , and are further away from the nucleus
weaker electrostatic foa between nucleus and electron pair in a covalent bond,
electronegativity decreases
describe the trend of melting point down group 2
group 2 metals have giant metallic lattice structure with strong metallic bonds between the metal cations and sea of delocalised electrons.
down the group, the size of cations increase and charge density decreases
weaker electrostatic foa between the cations and sea of delocalised electrons
thus less energy is required to overcome the weaker metallic bonds
mp decreases
describe the reducing power/chemical reactivity down group 2
down the group,
atomic radius increases thus there is weaker electrostatic foa between the nucleus and valence electrons
and metal atoms lose their valence electrons to form M²+ cations more easily
there is a greater tendency to be oxidised hence reducing power/chemical reactivity increases down the group
the E naught value of group 2 metals becomes more negative down the group,
showing that the tendency for group 2 metals to be oxidised to M2+ increases,
thus the reducing power and reactivity of group 2 metals increases down the group
what factors affect the stability of group 2 oxyanions/carbonates?
1) size and charge of cation → charge density of cations
charge density directly proportional to q+/r+
2) size of oxyanion
what do group 2 carbonates produce upon thermal decomposition?
metal oxide and CO2 (g)
describe and explain the trend of thermal stability of group 2 carbonates.
down the group,
the size of cations increases while the NC remains constant
the charge density of cations decrease, and the electron cloud of CO3²- is distorted/polarised to a smaller extent
thus C-O bond is weakened to a smaller extent and
thermal stability of group 2 carbonates increases down the group
describe the structure and bonding of group 17 elements.
the group 17 elements exist as diatomic molecules with
simple molecular structure and
weak id-id interactions between the non-polar X2 molecules
describe the trend of volatility down group 17.
down the group,
the no. of electrons and hence size of electron cloud increases,
thus polarisability of electron cloud increases
and more energy is needed to overcome the stronger intramolecular id-id interactions,
thus boiling point increases / volatility decreases down the group
describe the colours and states of group 17 elements at rep
Cl2 → greenish yellow gas
Br2 → reddish-brown liquid
I2 → black solid
describe the trend of oxidising power/reactivity of group 17 elements.
down the group,
atomic radius increases, thus there is decrease in tendency for X2 to accept electrons
and decrease in tendency for X2 to be reduced to X-
thus oxidising power of halogens decrease down the group
E naught value becomes less positive down the group
thus halogens have less tendency to be reduced
and have weaker oxidising power
describe structure and bonding of hydrogen halides HCl, HBr, HI.
they have
simple molecular structure
weak id-id and pd-pd interactions between the polar HX molecules
describe the trend of boiling point of group 17 hydrides (HCl, HBr, HI)
down the group,
no. electrons increase thus polarisability of electron cloud increases
more energy required to overcome the stronger id-id interactions
thus boiling point increases
describe the trend of thermal stability of group 17 hydrides (HCl, HBr, HI)
down the group,
size of halogen atoms increase
thus effectiveness of orbital overlap between H and X atoms decreases,
Bond strength of H-X decreases, thus BE of H-X decreases
less energy required to break H-X bond
thermal stability decreases