biochemistry
0.0(0)
Card Sorting
1/238
Earn XP
Last updated 2:33 AM on 2/9/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
239 Terms
1
New cards
irritability
the capacity of cells to sense their environment, to learn from the world around them, and to adapt by recalling and implementing effective “coping” strategies. Also includes the ability to communicate both within and among organisms.
2
New cards
Motility
the capacity to generate the molecular-scale forces that are needed for cells and organisms to move, for metabolites to be transported across membranes, for sub cellular components to be translocated, for chromatin to be dynamically restructured, for muscle contraction, cell crawling etc.
3
New cards
Reproduction
the capacity to generate entire organisms, thereby renewing their vitality, while correcting any errors.
4
New cards
Sources of redox energy
solar energy: Earth receives 4 x 10^24 Joules/year of sunlight
Geochemical: mainly oxidation of sulfur and minerals spewed from volcanoes & thermal vents; pre-dated photosynthesis
Geochemical: mainly oxidation of sulfur and minerals spewed from volcanoes & thermal vents; pre-dated photosynthesis
5
New cards
Sources of thermal energy
Sun’s infrared light
radioactivity: 40-50% of Earth’s surface heat comes from nuclear fission deep within its core
Steady-state balance: rate of heating = rate of cooling- important to create temperate environment that’s conducive for life
radioactivity: 40-50% of Earth’s surface heat comes from nuclear fission deep within its core
Steady-state balance: rate of heating = rate of cooling- important to create temperate environment that’s conducive for life
6
New cards
Gravitational energy
Earth’s rotation- day/night cycles and tides
Its near-circular orbit- near-constant temperature and moderate seasons
Earth’s tilt- attenuation of damaging UV light striking Earth’s surface
Its near-circular orbit- near-constant temperature and moderate seasons
Earth’s tilt- attenuation of damaging UV light striking Earth’s surface
7
New cards
Capturing Solar Energy: photosynthesis
Life thrived, once there were pigments capable absorbing the suns considerable output of electromagnetic radiation
8
New cards
NADH- Nature’s hydride battery
by accepting electrons as hydride ions, NAD+ becomes NADH, thereby allowing cells to store/manage chemical energy
9
New cards
Hydride anion
a hydrogen atom with one more electon
H-
H-
10
New cards
Draw NADH/NAD+ oxidation/reduction
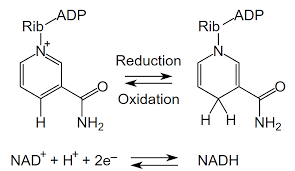
11
New cards
Oxidative Phosphorylation
NADH oxidation supplies energy to make 3 mol ATP from 3 mol ADP & 3 mol phosphate
12
New cards
bioenergetics- biological thermodynamics
defined as the systematic study of energy-transducing processes in cells, with the goal of learning:
how cells extract energy from their environment
how they use this energy to synthesize biomolecules and/or drive energy-requiring processes
how the efficiency of biological pathways changes in disease or metabolic stress
how cells extract energy from their environment
how they use this energy to synthesize biomolecules and/or drive energy-requiring processes
how the efficiency of biological pathways changes in disease or metabolic stress
13
New cards
Biology obeys the laws of thermodynamics- NO EXCEPTIONS
1st law- energy may change form or may be transported, BUT energy is not created or destroyed
2nd law- the entropy of the universe always increases
2nd law- the entropy of the universe always increases
14
New cards
equilibrium thermodynamics is path-independent
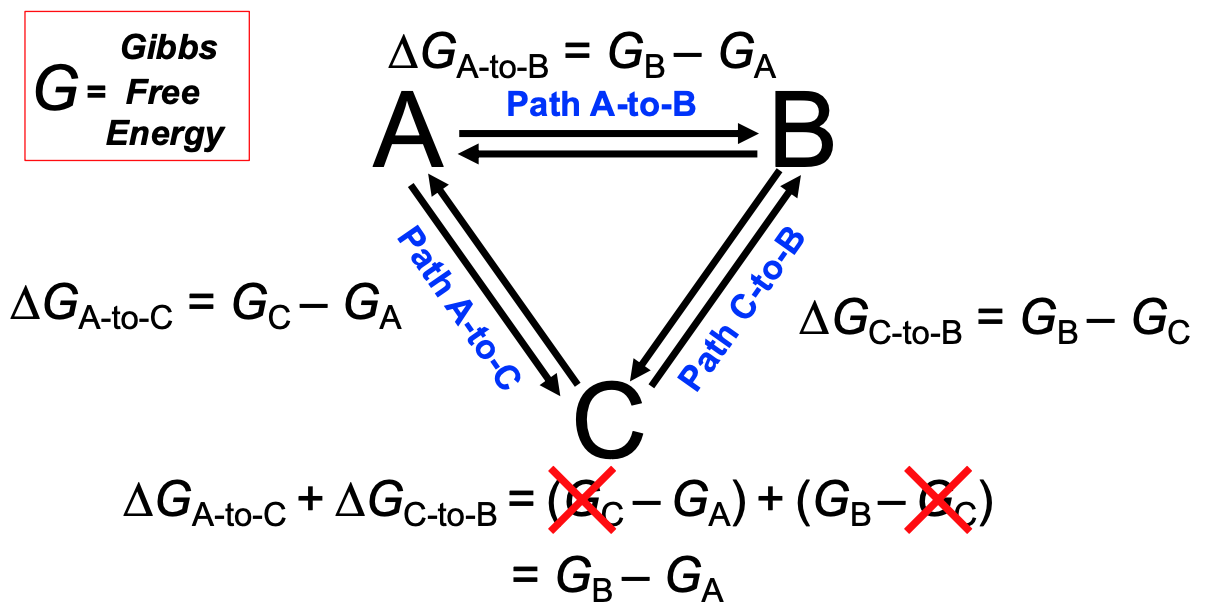
15
New cards
Gibbs Energy
measure of the work that a system can perform at constant temperature and pressure
16
New cards
key gibbs free equations \*\*\*\*
*delta****G*****°** = delta*H* - *TdeltaS*
delta***G*****°**= -RTlnKeq
delta***G*****°**= -RTlnKeq
17
New cards
DeltaH**°**
standard enthalpy change- measures the standard state change in the internal energy content, as reflected in the number and stability of all bonds and bonding interactions in the products versus reactants
18
New cards
DeltaS**°**
Standard entropy change- measures the standard change in the disorder of a chemical reaction
19
New cards
Enthalpy
total energy of a reacting system plus any available “PV” work: deltaH= H(products)- H(reactants)
20
New cards
DeltaH>0
heat content of the covalent & non-covalent bonds in the products is MORE than the heat content of covalent & non-covalent bonds of the reactants
Heat is absorbed to form products
Heat is absorbed to form products
21
New cards
DeltaH= 0
heat content of the covalent & non-covalent in the products is same as the heat content of the covalent & non-covalent bonds of the reactants
No heat is liberated or absorbed, upon the formation of products
No heat is liberated or absorbed, upon the formation of products
22
New cards
DeltaH
we say that the heat content of the covalent & non-covalent bonds in the products is less than the heat content of the covalent & non-covalent bonds of the reactants
Heat is liberated when products form
Heat is liberated when products form
23
New cards
Entropy
represents the fraction of a system’s energy that, as a result its randomness, cannot be converted into useful work
TdeltaS = T (S(products)- S(reactants))
TdeltaS = T (S(products)- S(reactants))
24
New cards
DeltaS>0
there is more disorder in the products than the reactants
overall randomness increases as products form
overall randomness increases as products form
25
New cards
DeltaS=0
overall disorder does not change when products form
overall randomness is unchanged as products form
overall randomness is unchanged as products form
26
New cards
DeltaS
overall disorder of the products is less than the overall disorder of those of the reactants
overall randomness decreases as products form
overall randomness decreases as products form
27
New cards
Standard Free Energy Change, Delta***G*****° ******
Reacting systems always move towards equilibrium
at equilibrium, forward and reverse reaction rates are equal
concentrations of reactants and products at equilibrium define the reaction’s equilibrium constant Keq
at equilibrium, forward and reverse reaction rates are equal
concentrations of reactants and products at equilibrium define the reaction’s equilibrium constant Keq
28
New cards
How Keq and DeltaG**°** are related
DeltaG**°** is a measure of how far a reaction must go to reach its final or equilibrium position
each chemical reaction has its own DeltaG**°** value
each chemical reaction has its own DeltaG**°** value
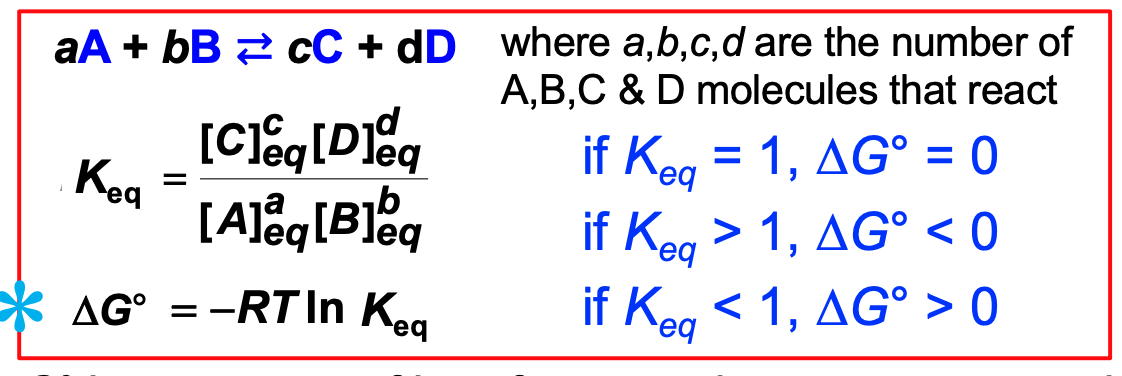
29
New cards
Biochemical Standard State Conditions
In chemistry Keq and DeltaG**°** are thermodynamic parameters that apply to chemical standard state conditions
Such conditions are not convenient for studying biochemical processes
Renormalized parameters where Keq’ and DeltaG**°**'‘ (Molarity, ph=7, incorporate H2O concentration and T=37 degrees celsius, P=1 atm and 1-2mM free MG2+)
always use these and indicated by (‘)
Such conditions are not convenient for studying biochemical processes
Renormalized parameters where Keq’ and DeltaG**°**'‘ (Molarity, ph=7, incorporate H2O concentration and T=37 degrees celsius, P=1 atm and 1-2mM free MG2+)
always use these and indicated by (‘)
30
New cards
The actual free energy change (DeltaG) is the thermodynamic driver within cells (No little circle)
the free energy change for a reaction depends on the actual concentrations of substrates and products, as well as the temperature
Intracellular concentrations are hardly constant, they depend on diet, exercise, and even disease
Intracellular concentrations are hardly constant, they depend on diet, exercise, and even disease
31
New cards
Delta G uses the actual concentrations
if DeltaG
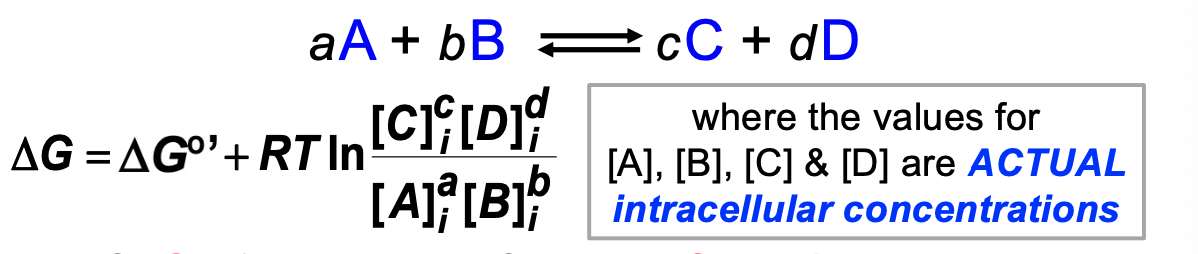
32
New cards
Key differences between DeltaG and DeltaG**°’ *****
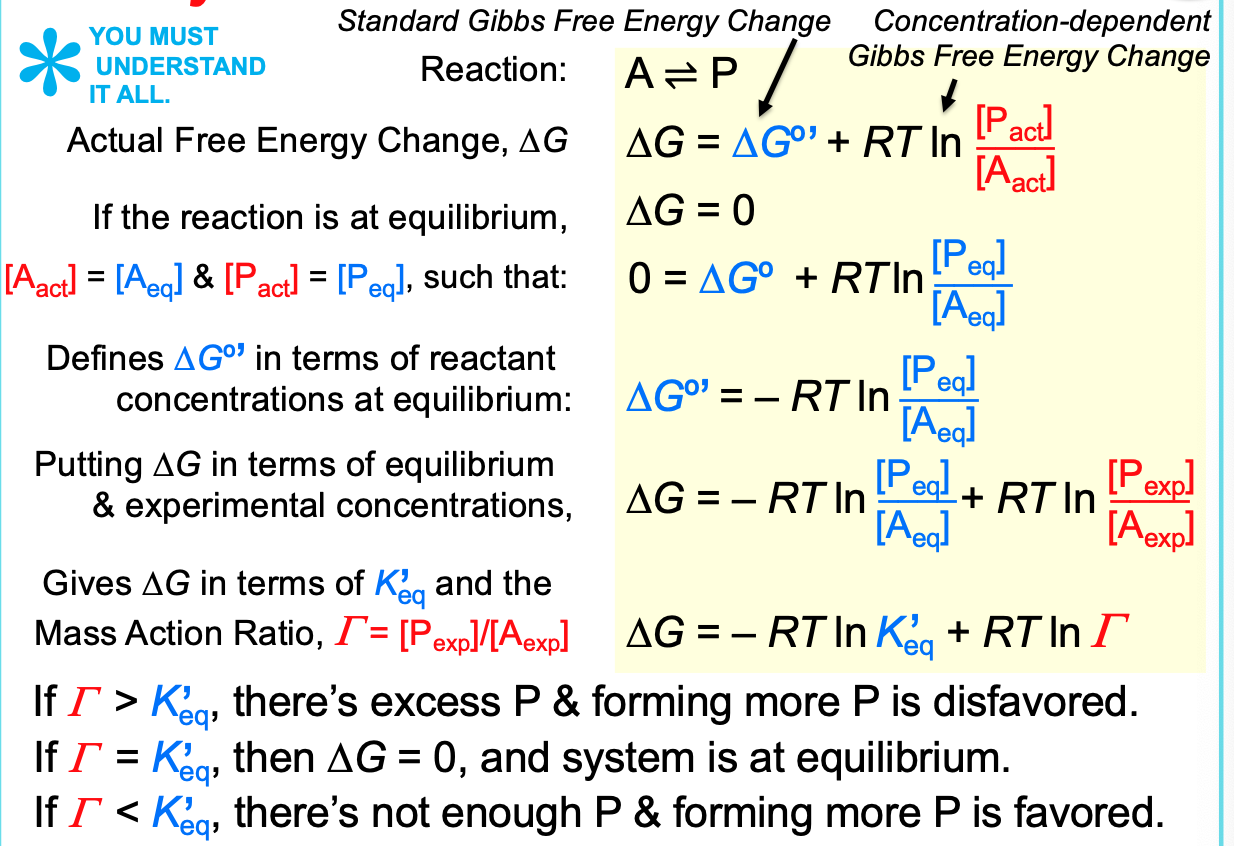
33
New cards
How we measure metabolites in situ?
Wollenberger Tongs
Consists of 2 aluminum blocks, that are joined by a hinge and a long arm with finger rings
They are brought to a very cold temperature
Then without interrupting blood flow and organ/tissue is squeezed between the plates,
The very low temp, the high rate of heat transfer to the metal plates results in freezing very fast
can analyze samples by many techniques
Consists of 2 aluminum blocks, that are joined by a hinge and a long arm with finger rings
They are brought to a very cold temperature
Then without interrupting blood flow and organ/tissue is squeezed between the plates,
The very low temp, the high rate of heat transfer to the metal plates results in freezing very fast
can analyze samples by many techniques
34
New cards
Isolated system
no energy or mass is exchanged between system and surroundings
35
New cards
closed system
energy is exchanged, but mass is not
36
New cards
open system
both mass and energy are exchanged with the surroundings
37
New cards
Hydrogen bond
stable interaction occurring when two electronegative atoms share a hydrogen bond
H-bonds are constantly made and broken
liquid water is thus a flickering collection of W5 (5 H20 molecules) with extremely little W1
H-bonds are constantly made and broken
liquid water is thus a flickering collection of W5 (5 H20 molecules) with extremely little W1
38
New cards
H2O readily forms hydrogen bonds \*\*\*
fundamentally its an acid/base interaction
stronger when all three atoms are aligned
stronger when all three atoms are aligned
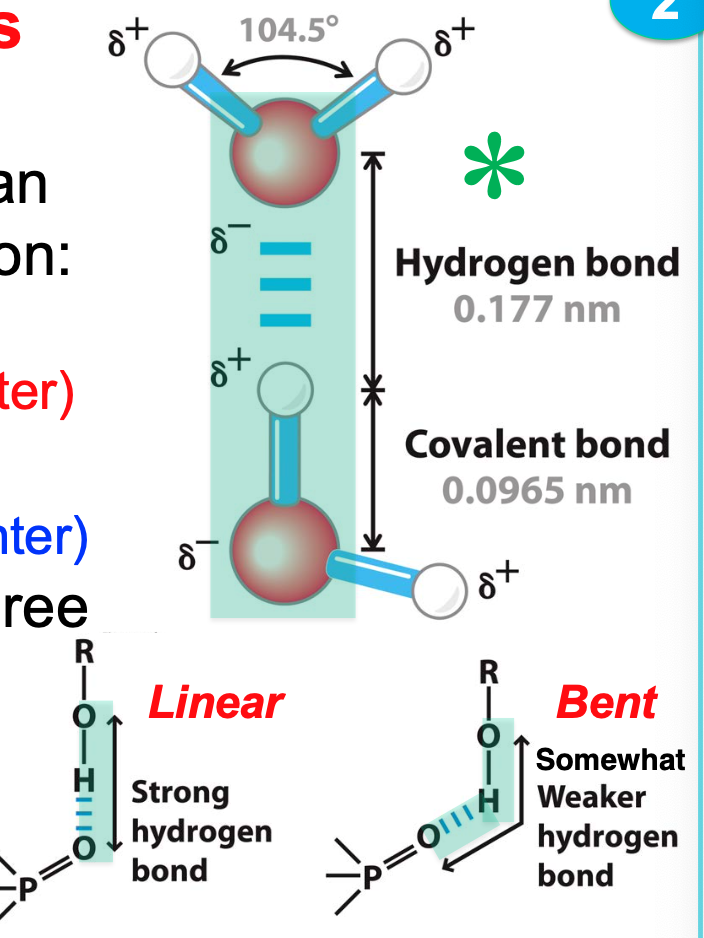
39
New cards
Waters key properties
High heat capacity
High B.P. relative to its molecular weight
high viscosity for its molecular weight
strong interactions with ionic molecules
special interactions with nonpolar molecules
liquid h2o has 3.4 h-bonds on average
ice has 4 h-bonds
High B.P. relative to its molecular weight
high viscosity for its molecular weight
strong interactions with ionic molecules
special interactions with nonpolar molecules
liquid h2o has 3.4 h-bonds on average
ice has 4 h-bonds
40
New cards
H2O deactivates nucleophilic reactions
nucleophilic reactions work best within polar organic solvents, DMF or THF
H2O greatly retards nucleophilic reactions- water crowds electron rich and electron deficient reactants, thereby deactivating them
While seemingly disadvantageous, very beneficial to life
uncatalyzed reactions are extremely slow under physiologic conditions-life requires enzyme catalysis
H2O greatly retards nucleophilic reactions- water crowds electron rich and electron deficient reactants, thereby deactivating them
While seemingly disadvantageous, very beneficial to life
uncatalyzed reactions are extremely slow under physiologic conditions-life requires enzyme catalysis
41
New cards
Glucose phosphorylation
enzymes increase reactivity so metabolism can occur under otherwise unfavorable conditions
catalyzed reactions reaches equilibrium in 2-3 seconds where uncatalyzed reaction takes 1 billion sec
catalyzed reactions reaches equilibrium in 2-3 seconds where uncatalyzed reaction takes 1 billion sec
42
New cards
Vital role enzymes play
enzymes bind and dehydrate reactants: increase local concentration of reactants, orient substrates to maximize reactivity, stabilize reaction transition states, make reactions faster
enzymes operate as on/off switches: insignificant reaction occurs in absence of the enzyme, little or no toxic side reactions, highly effective regulation
enzymes operate as on/off switches: insignificant reaction occurs in absence of the enzyme, little or no toxic side reactions, highly effective regulation
43
New cards
Acid/base properties of water
undergoes rapid H+ dissociation/reassociation
In reality there is no free H+; instead H+ is transferred to a neighboring H2O, thereby forming hydronium ion H3O+
In reality there is no free H+; instead H+ is transferred to a neighboring H2O, thereby forming hydronium ion H3O+
44
New cards
Proton hopping
in liquid H2O protons hop
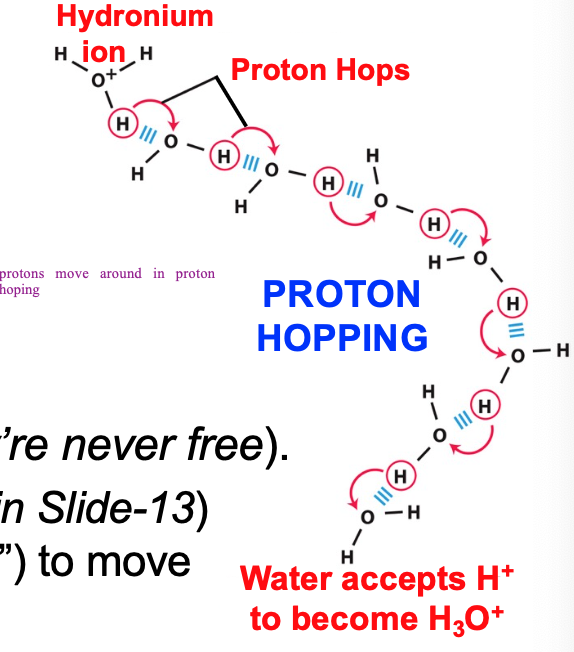
45
New cards
autoprotolysis
a proton is transferred between 2 identical molecules; one H2O acts as Bronsted acid (proton donor) and the other H2O molecule acts as Bronsted base (proton acceptor)
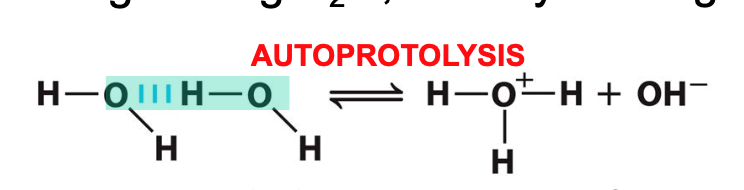
46
New cards
pH equations
pH= -log10 \[H3O+\]
Kw= \[H3O+\]\[OH-\]= 10^-14 M^2
pH meters don’t measure \[H+\] but proton activity
pH meters must be standardized with solutions of known concentration and activity, therefore the measured pH of pure water is about 7 not exactly 7
Kw= \[H3O+\]\[OH-\]= 10^-14 M^2
pH meters don’t measure \[H+\] but proton activity
pH meters must be standardized with solutions of known concentration and activity, therefore the measured pH of pure water is about 7 not exactly 7
47
New cards
deriving the Henderson-Hasselbalch Equation \*\*\*\*
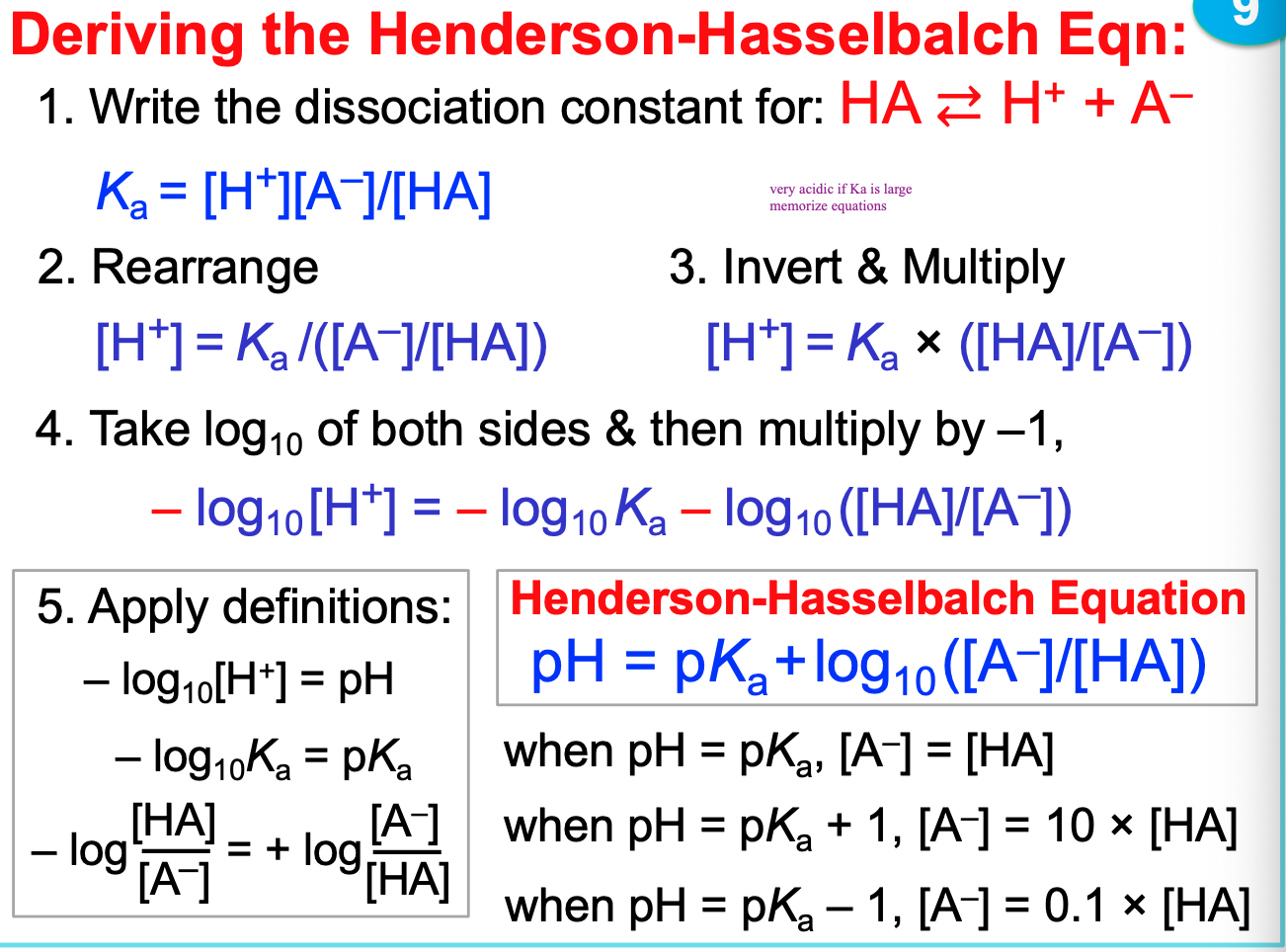
48
New cards
pH is important in biochemistry
many metabolites have acid or base groups
enzymes have acids and bases on active sites
DNA is polyanion & histones are polycationic porteins
enzymes have acids and bases on active sites
DNA is polyanion & histones are polycationic porteins
49
New cards
Buffers
weak acids and weak bases that are partially dissociated helping to stabilize pH
buffers obey Le Chateliers principle
stabilize nearly constant pH
stabilize structures of cells, proteins, nucleic acid
biochemists use buffers to stabilize biological structure to assure reproducibility of their experiments
buffers obey Le Chateliers principle
stabilize nearly constant pH
stabilize structures of cells, proteins, nucleic acid
biochemists use buffers to stabilize biological structure to assure reproducibility of their experiments
50
New cards
Le Chatelier’s Principle
dynamic systems compensate to an applied stress
51
New cards
Buffer titration curve
buffers are most effective at pH near their pKa where there’s an equally large pool of both weak acid its conjugate base'
effective buffer zone is shown in blue box
pKa is where concentration of conjugate base equals concentration of acid
effective buffer zone is shown in blue box
pKa is where concentration of conjugate base equals concentration of acid
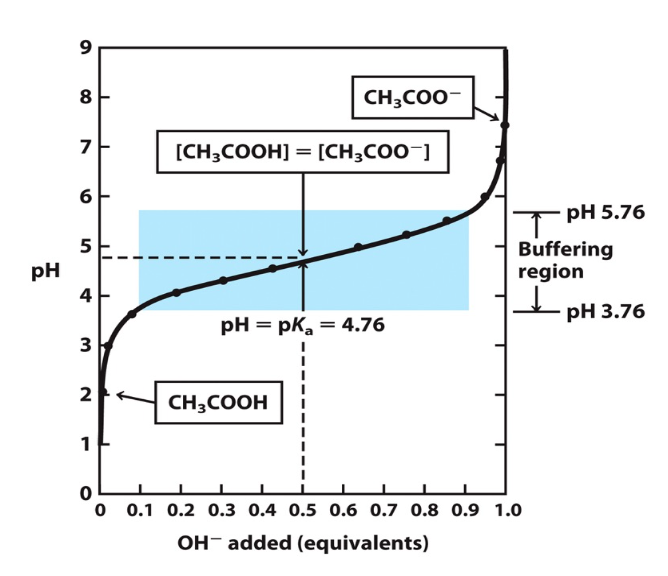
52
New cards
HCO3-/CO2 buffer system
most important buffer in human acid-base homeostasis
normal arterial plasma pH is 7.4 +/- 0.05 in humans
fluctuations are usually lethal
relative concentrations of HCO3- and CO2 determine plasma pH
uncatalyzed rate is slow and must be accelerated by an enzyme known as carbonic anhydrase
normal arterial plasma pH is 7.4 +/- 0.05 in humans
fluctuations are usually lethal
relative concentrations of HCO3- and CO2 determine plasma pH
uncatalyzed rate is slow and must be accelerated by an enzyme known as carbonic anhydrase
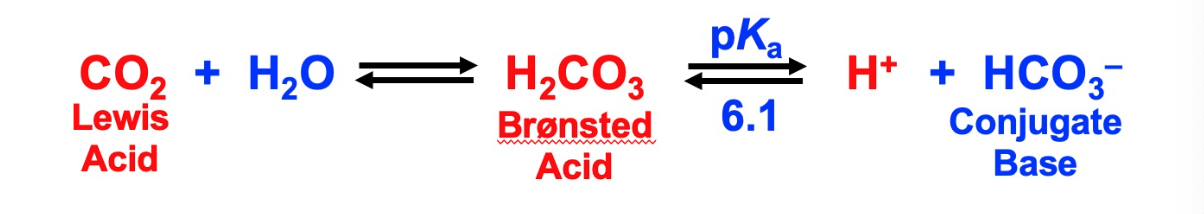
53
New cards
Respiratory compensation
ability of our body to adjust its breathing rate to modify circulating CO2
with pKa of 6.1 would seem that HCO3-/CO2 system would be an ineffective buffer at ph 7.4 however its highly effective because body has respiratory compensation
with pKa of 6.1 would seem that HCO3-/CO2 system would be an ineffective buffer at ph 7.4 however its highly effective because body has respiratory compensation
54
New cards
acidosis
low blood pH, hyperventilation drives CO2 out of the body and reduces \[H+\] returning blood to pH 7.4
![low blood pH, hyperventilation drives CO2 out of the body and reduces \[H+\] returning blood to pH 7.4](https://knowt-user-attachments.s3.amazonaws.com/2abd2f120c0e4093b7d5f72e9f0a9b4d.jpeg)
55
New cards
alkalosis
high blood pH, rebreathing pushes CO2 into the body, and increases \[H+\] returning blood pH to 7.4
![high blood pH, rebreathing pushes CO2 into the body, and increases \[H+\] returning blood pH to 7.4](https://knowt-user-attachments.s3.amazonaws.com/6912ee02aa0d4b8ca110bcdcd0006cff.jpeg)
56
New cards
Osmotic pressure of aqueous solutions
minimum pressure that must be applied to a solution to prevent the inward flow of pure water across a semipermeable membrane
measures the tendency of a solution on one side of semipermeable membrane to draw in pure solvent from the other side of a semipermeable membrane
measures the tendency of a solution on one side of semipermeable membrane to draw in pure solvent from the other side of a semipermeable membrane
57
New cards
amino acid composition
always have amino group on an alpha carbon (⍺)
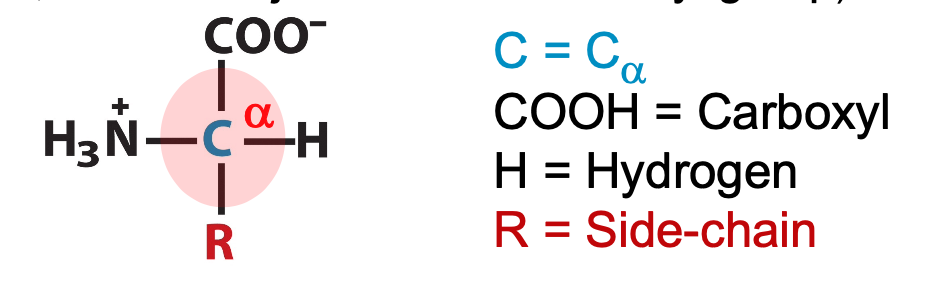
58
New cards
stereoisomerism is based on fischer projection for L-glyveradehyde
only alpha amino acids are found in proteins not beta
carboxyl group is on the top and side chain on bottom with hydrogen on right of alpha carbon
carboxyl group is on the top and side chain on bottom with hydrogen on right of alpha carbon
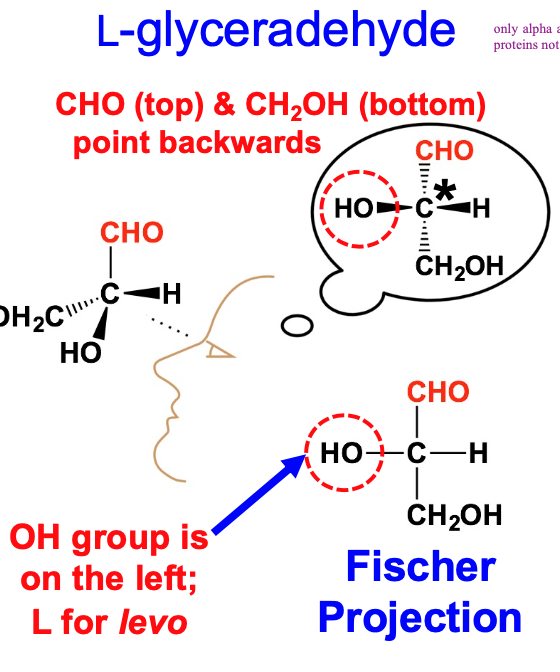
59
New cards
Amino acids with nonpolar aliphatic R groups
Glycine, Alanine, Valine, Leucine, Isoleucine, Methionine, and Proline
60
New cards
Amino acids with aromatic side-chains
Phenylalanine, Tyrosine, and Tryptophan
absorb UV light
absorb UV light
61
New cards
Amino acids with positively charged side-chains
Lysine, Arginine, and Histidine
Have significant side-chain positive charge at pH 7
Histidine is the only basic AA with an ionizable group near neutrality: can serve both as H+ donor or H+ acceptor in enzyme-catalyzed reactions
Have significant side-chain positive charge at pH 7
Histidine is the only basic AA with an ionizable group near neutrality: can serve both as H+ donor or H+ acceptor in enzyme-catalyzed reactions
62
New cards
Amino acids with negatively charged R groups
Aspartate and Glutamate
63
New cards
Amino Acids with uncharged polar side-chains
Serine, Threonine, Cysteine, Asparagine, and Glutamine
Polar R-groups from H-bonds with water
Polar R-groups from H-bonds with water
64
New cards
Cysteine & Cystine
Free SH group in cysteine ionizes and is largely undissociated at physiologic pH
Think of cysteine having more electrons, a characteristic of a reduced compound
cystine is mainly present in circulation
Think of cysteine having more electrons, a characteristic of a reduced compound
cystine is mainly present in circulation
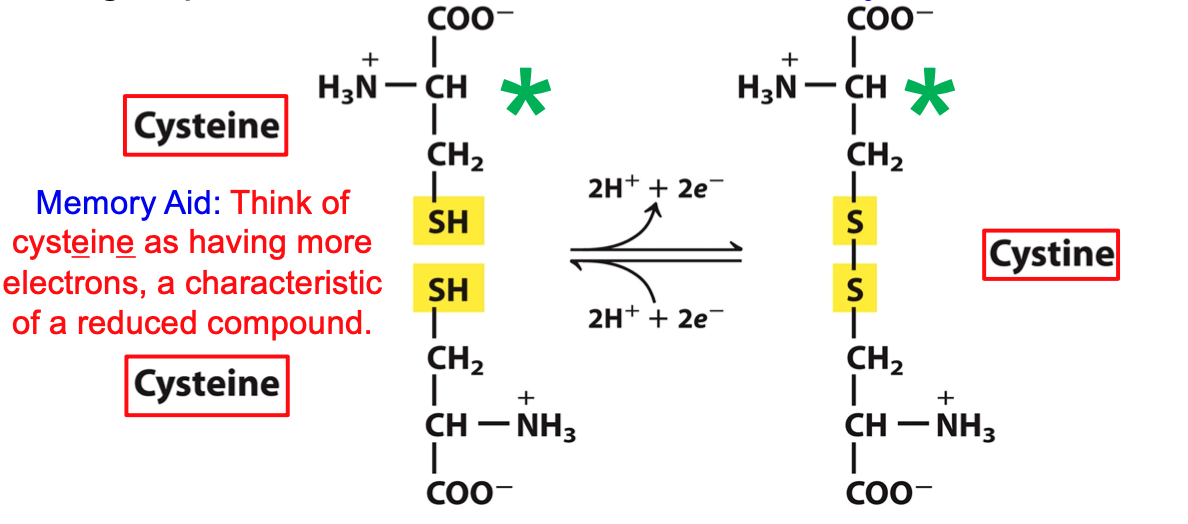
65
New cards
Antibody structure is stabilized by S-S bonds
without them- loss of light chain from bloodstream, no antigen recognition and no cross-linking action (agglutination- react with two molecules)
without them- loss of light chain from bloodstream, no antigen recognition and no cross-linking action (agglutination- react with two molecules)
66
New cards
Amino acids are ampholytes
contain both acidic and basic groups and therefore form zwitterion
in between more and less protonated forms
in between more and less protonated forms
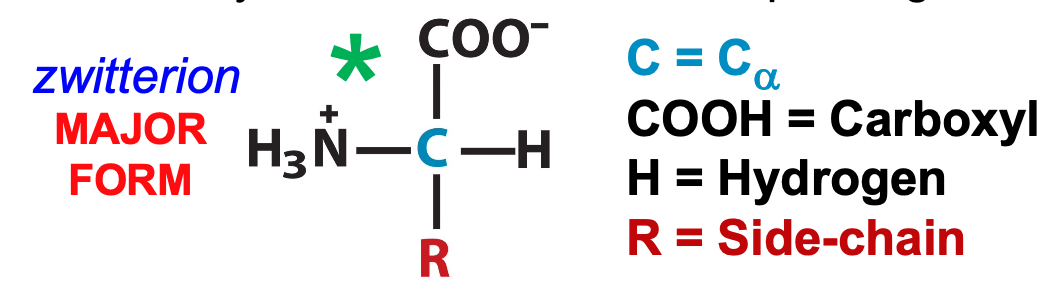
67
New cards
Ionization of amino acids
charge on amino acid depends on solution pH
we begin at low pH (fully protonated) and add OH- (removing acidic protons first)
we begin at low pH (fully protonated) and add OH- (removing acidic protons first)
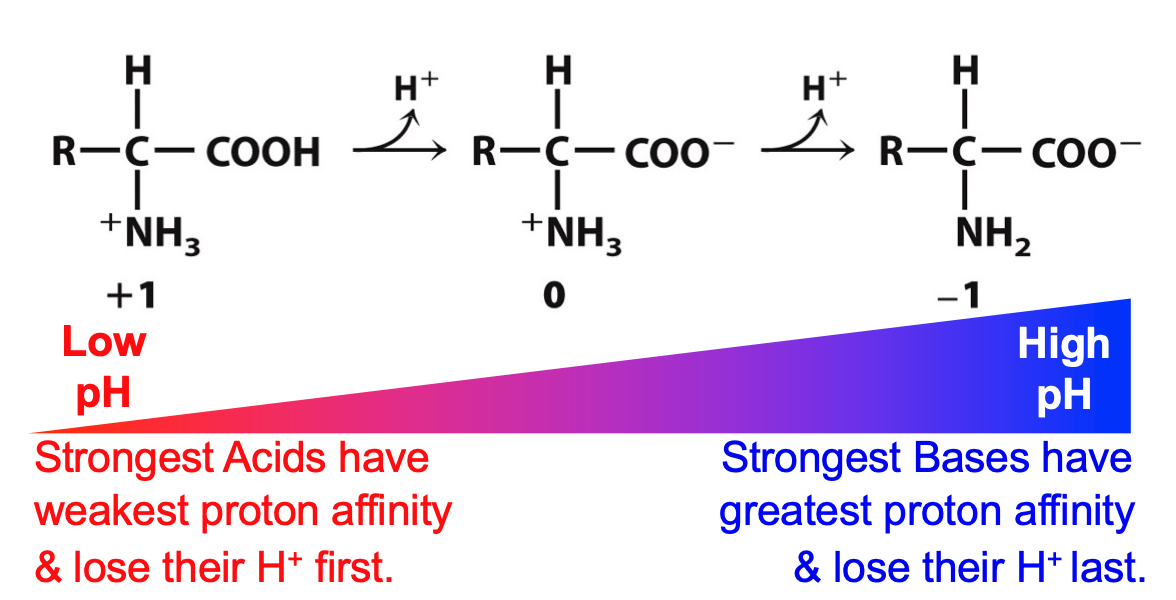
68
New cards
isoelectric point of amino acids
defined as pH where amino acid has average charge of 0
69
New cards
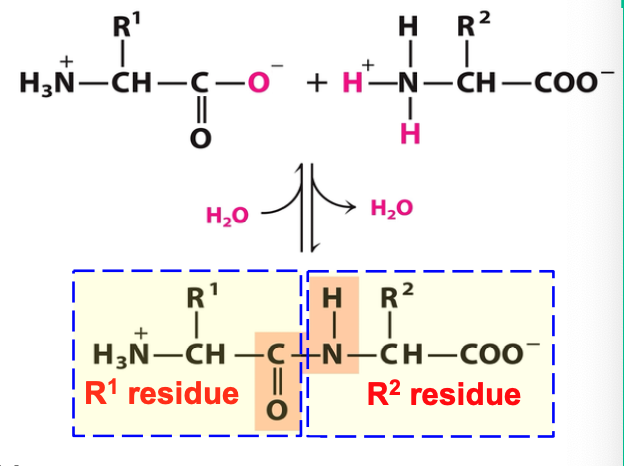
Peptide bond
one H2O molecule is lost in forming peptide bond
what remains of each AA is called a residue
N-terminus (written on left) and C-terminus (written on right) are unmodified -NH2 and -COOH
peptides formation is by ribosomes
what remains of each AA is called a residue
N-terminus (written on left) and C-terminus (written on right) are unmodified -NH2 and -COOH
peptides formation is by ribosomes
70
New cards
Solid-phase peptide synthesis
Exploits solid-phase resin, on which the peptide is progressively elongated by:
1. loading one amino acid at a time
2. washing and deprotecting to generate newly reactive functional groups
3. chemical coupling to form bond and
4. release of final peptide from resin
1. loading one amino acid at a time
2. washing and deprotecting to generate newly reactive functional groups
3. chemical coupling to form bond and
4. release of final peptide from resin
71
New cards
Vastness of protein sequence space
20^n
for tetrapeptide 20^4= 160,000
for 400-residues 20^400= 10^520
for tetrapeptide 20^4= 160,000
for 400-residues 20^400= 10^520
72
New cards
How to determine isoelectric point of peptides
1. start with fully protonated form
2. remove protons one at a time until you get to +1 form
3. add up the next two pKa values & divide by 2
73
New cards
Determining Protein Sequences
Amino acid sequence is determined in two ways
1. cDNA sequencing
2. Enzymatic/chemical fragmentation, followed by fragment separation and automated sequencing
not good to sequence DNA want to sequence amino acids
have a way to remove a single amino acid at a time-accumulate little bit of each amino acid cleaving and can become very messy
1. cDNA sequencing
2. Enzymatic/chemical fragmentation, followed by fragment separation and automated sequencing
not good to sequence DNA want to sequence amino acids
have a way to remove a single amino acid at a time-accumulate little bit of each amino acid cleaving and can become very messy
74
New cards
Trypsin cleaves where
carboxyl side of Arg & Lys
75
New cards
Chymotrypsin cleaves where
carboxyl side of Phe (F), Tyr (Y) and Try (W)
76
New cards
How are amino acid sequences written
always with N-terminus on the left, cleavage occurs “AFTER” the specific residue, freeing its COOH group
77
New cards
Cyanogen Bromide (cNBr) Fragmentation
cleaves only after Met to produce two peptides, leaving homoserine lactone lactone at its C-terminus
cleanly fragments protein into a set of peptides
has no effect, if Methionine is on C-terminus
cleanly fragments protein into a set of peptides
has no effect, if Methionine is on C-terminus
78
New cards
sequencing unknown AA sequence
most proteins have 400-500 amino-acid residues-not possible to chemically sequence entire things so have to cleave protein into smaller fragments
illustrate with 28-residue peptide
1. treat separate samples of pure peptide with Trypsin, Chymotrypsin or CNBr
2. isolate each fragment by chromatography
3. Chemically sequence each fragment
NOTE: we wont know the order of the fragments. So we must use multiple fragmentation methods to obtain sets of overlapping sequences
illustrate with 28-residue peptide
1. treat separate samples of pure peptide with Trypsin, Chymotrypsin or CNBr
2. isolate each fragment by chromatography
3. Chemically sequence each fragment
NOTE: we wont know the order of the fragments. So we must use multiple fragmentation methods to obtain sets of overlapping sequences
79
New cards
Edman Sequencing
repetitive removal of AA from N-terminus and subsequent identification
R is different for each amino acid
PTH-AA is identified by gas or liquid chromatography
Process is repeated over and over for 40-50 cycles
automated sequencing of 5-20 ug peptide/protein
R is different for each amino acid
PTH-AA is identified by gas or liquid chromatography
Process is repeated over and over for 40-50 cycles
automated sequencing of 5-20 ug peptide/protein
80
New cards
Why bother with fragment sequencing?
REMEMBER: often interested in the sequence of the physiologically active form of the protein
Many genes give rise to proteins of different AA sequences, depending on the tissue in which they are expressed
mRNA can be spliced, removing certain sequences and altering the actual sequence of the expressed protein
many proteins are post-translationally modified specialized amino acids
many proteins are post-translationally cleaved, such that the final amino acid sequence does not match sequence encoded in the DNA sequence
best to do both protein and DNA sequencing
Many genes give rise to proteins of different AA sequences, depending on the tissue in which they are expressed
mRNA can be spliced, removing certain sequences and altering the actual sequence of the expressed protein
many proteins are post-translationally modified specialized amino acids
many proteins are post-translationally cleaved, such that the final amino acid sequence does not match sequence encoded in the DNA sequence
best to do both protein and DNA sequencing
81
New cards
Why are noncovalent interactions so vital?
strengths of noncovalent bonding interactions are “tunable”- meaning their weaker energies depend on many factors, such as hydration, electrostatics, polarity etc.
they are made and/or broken readily
they determine protein structure and function; enzyme specificity; DNA structure and function; membrane formation and stability; and nearly every other vital process in living systems
they are made and/or broken readily
they determine protein structure and function; enzyme specificity; DNA structure and function; membrane formation and stability; and nearly every other vital process in living systems
82
New cards
role of weak noncovalent interactions
four basic types
individually weak, the cumulative effect can be very significant
allow proteins and nucleic acids to fold and unfold on a biologically relevant time-scale
individually weak, the cumulative effect can be very significant
allow proteins and nucleic acids to fold and unfold on a biologically relevant time-scale
83
New cards
H-bonds in Nature
Enzyme-substrate interactions
Receptor-ligand interactions
RNA & DNA base-pairing
H-bonds are easy to make & just as easy to break (fast kinetics)
Receptor-ligand interactions
RNA & DNA base-pairing
H-bonds are easy to make & just as easy to break (fast kinetics)
84
New cards
Role of H-bonding in Proteins
H-bonding is NOT a major driving force in protein folding
formation of extended H-bond networks (especially in the form of ⍺-helix or beta-sheet structures) act as structure-organizing elements
⍺-helix or beta-sheet creates scaffold that helps dehydrate protein’s interior by positioning side-chains to maximize hydrophobic interactions
formation of extended H-bond networks (especially in the form of ⍺-helix or beta-sheet structures) act as structure-organizing elements
⍺-helix or beta-sheet creates scaffold that helps dehydrate protein’s interior by positioning side-chains to maximize hydrophobic interactions
85
New cards
Electrostatic interactions
attractive and repulsive forces
proteins have numerous ionizable groups
ionic groups tend to be on the surface of proteins
formation of ionic bond releases water
proteins have numerous ionizable groups
ionic groups tend to be on the surface of proteins
formation of ionic bond releases water
86
New cards
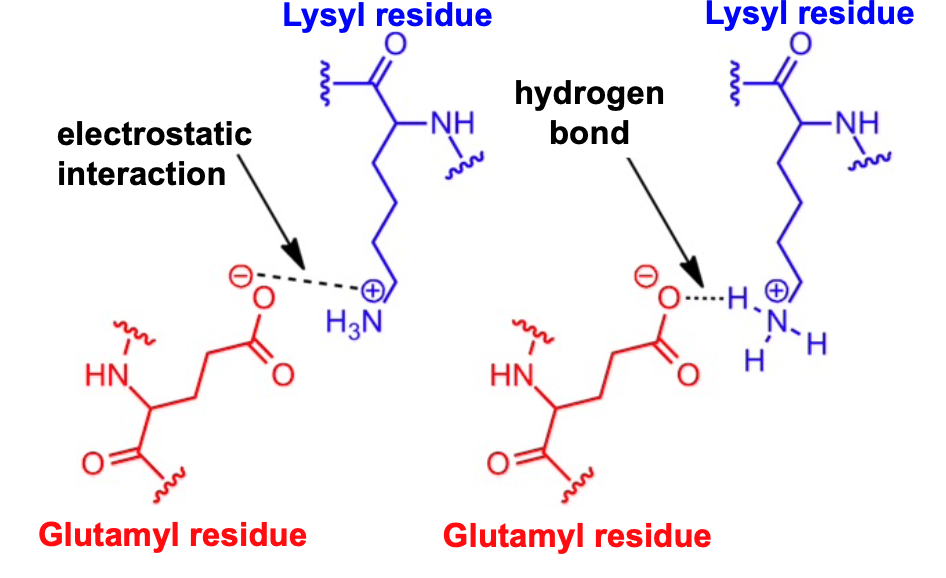
salt bridges
bonds between oppositely charged residues that are sufficiently close to each other to experience mutual electrostatic attraction
salt bridges are a combination of electrostatic interaction and H-bonding
salt bridges are a combination of electrostatic interaction and H-bonding
87
New cards
Hydrophobic interactions
strictly speaking they are NOT true bonds
strength of interaction is determined by number of organized water molecules released
also play a dominant role in stabilizing membrane bilayer
strength of interaction is determined by number of organized water molecules released
also play a dominant role in stabilizing membrane bilayer
88
New cards
Hydrophobic interactions and DeltaG
contrary to intuition, water bind fairly well to apolar groups such that deltaH(water-binding)<< 0 (deltaH(water-release)>>0)
release many water molecules greatly increasing randomness (deltaS(water-release)>>0)
temperature strongly influences deltaG- at lower temperatures deltaG can be unfavorable and at high deltaG can be favorable- it all depends on the value of TdeltaS not just deltaS
release many water molecules greatly increasing randomness (deltaS(water-release)>>0)
temperature strongly influences deltaG- at lower temperatures deltaG can be unfavorable and at high deltaG can be favorable- it all depends on the value of TdeltaS not just deltaS
89
New cards
Hydrophobicity and protein folding
protein folding decreases local entropy, but H2O release increases entropy- over deltaS is positive
side-chains participating in hydrophobic interactions can swivel, thereby facilitating protein folding
great variety of hydrophobic side-chains maximizes packing and minimized release of bound water
side-chains participating in hydrophobic interactions can swivel, thereby facilitating protein folding
great variety of hydrophobic side-chains maximizes packing and minimized release of bound water
90
New cards
van der Waals interactions
short-lived bond resulting from interactions of fluctuating dipoles on non-bonding electrons
net effect is a weak bond
operate over very short length scale for two spherical electrostatic fields
net effect is a weak bond
operate over very short length scale for two spherical electrostatic fields
91
New cards
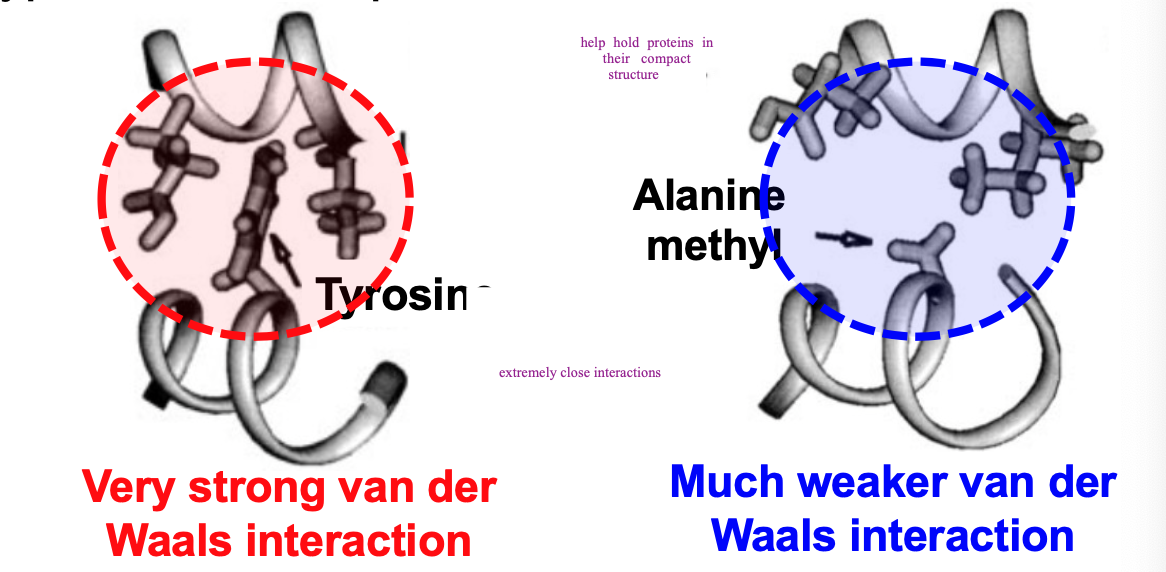
van der Waals and proteins
water interferes with van der Waals but within protein’s dehydrated interior, numerous van der Waals interactions contribute to protein stability
help hold proteins in their compact shape
help hold proteins in their compact shape
92
New cards
Pi-cation interactions
aromatic groups have highly delocalized pi-electron orbitals above and below plane of ring
orbitals often interact with quaternary ammonium ions to form stable bonds that are important in enzyme-substrate interactions and receptor-agonist interactions
in aqueous media, the cation-pi interaction is comparable (potentially stronger than) ammonium-carboxylate salt bridges
aromatic side chains of phenylalanine, tryptophan, tyrosine, histidine are capable of binding to cationic species, such as quaternized amino groups, metal ions, neurotransmitters and pharmaceutical agents
orbitals often interact with quaternary ammonium ions to form stable bonds that are important in enzyme-substrate interactions and receptor-agonist interactions
in aqueous media, the cation-pi interaction is comparable (potentially stronger than) ammonium-carboxylate salt bridges
aromatic side chains of phenylalanine, tryptophan, tyrosine, histidine are capable of binding to cationic species, such as quaternized amino groups, metal ions, neurotransmitters and pharmaceutical agents
93
New cards
Proteins role in cells
structural elements- collagen, histones, keratins
enzymes- catalysts for metabolism and signaling
antibodies- immune surveillance
receptors & channels- signal transduction
transporters- metabolites import/export
molecular motors- translocation
transcription factors- gene control
enzymes- catalysts for metabolism and signaling
antibodies- immune surveillance
receptors & channels- signal transduction
transporters- metabolites import/export
molecular motors- translocation
transcription factors- gene control
94
New cards
protein shape
spherical (preferred term is globular)
95
New cards
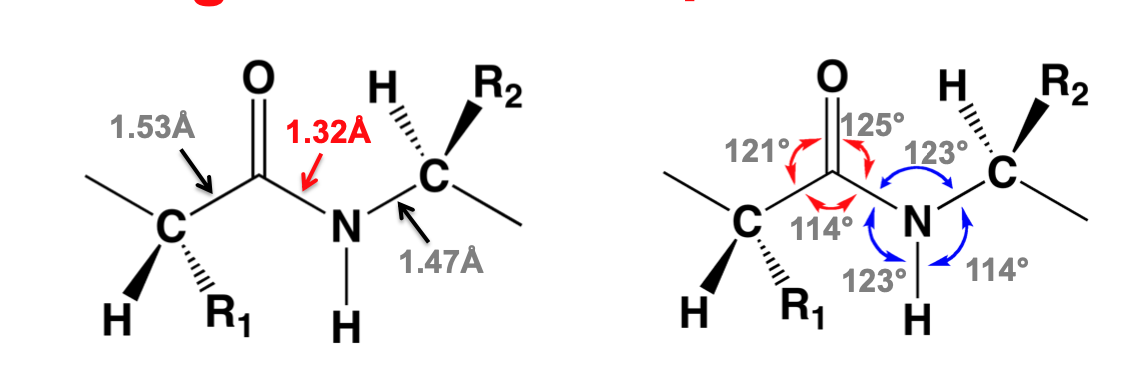
Peptide bond characteristics
consensus of bond-lengths and bond angles
C-N peptide bond length is consistently shorter than the C-N bond in most amides
\
C-N peptide bond length is consistently shorter than the C-N bond in most amides
\
96
New cards
Planarity of peptide bond \*\*\*
C-N bond has 40% double-bond character
resonance explains shorter bond-length
C=O and N-H atoms within peptide bond are approximately coplanar (very important)
resonance explains shorter bond-length
C=O and N-H atoms within peptide bond are approximately coplanar (very important)
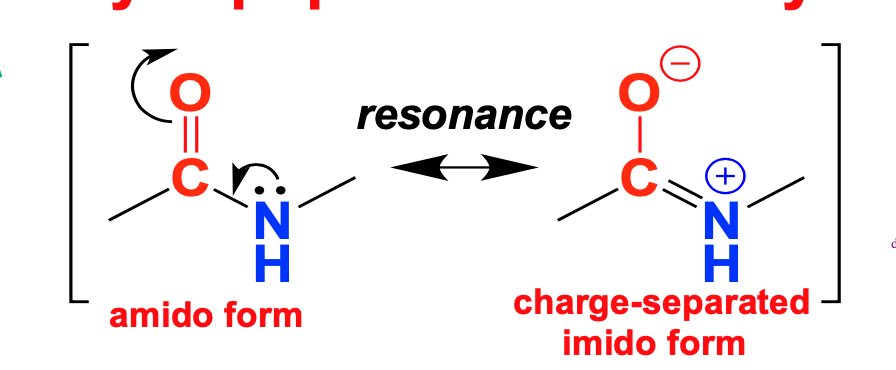
97
New cards
planarity of multiple AA/peptide bonds
each shaded bond system behaves as planar unit
allows proteins to fold rapidly- reduced freedom greatly facilitates this
amino acid side chains direct the folding of the nascent (newly formed) polypeptide into a functional protein and do much to stabilize that final conformation
allows proteins to fold rapidly- reduced freedom greatly facilitates this
amino acid side chains direct the folding of the nascent (newly formed) polypeptide into a functional protein and do much to stabilize that final conformation
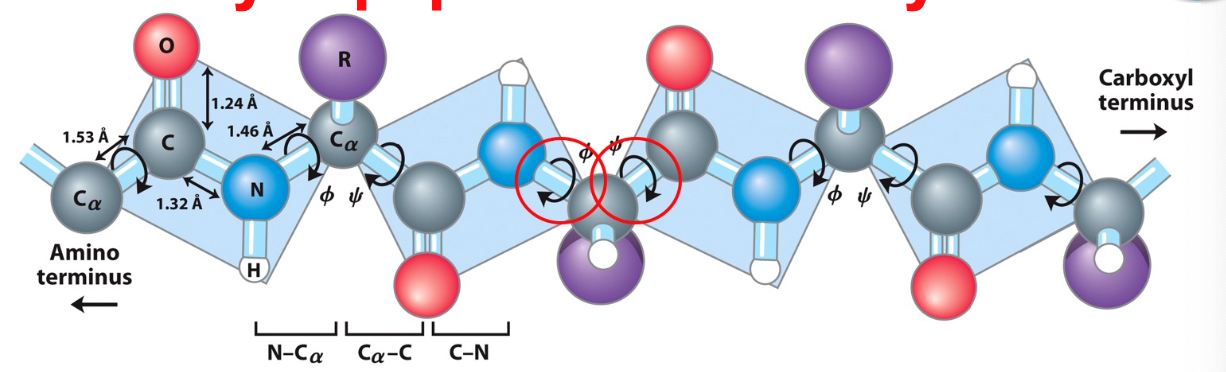
98
New cards
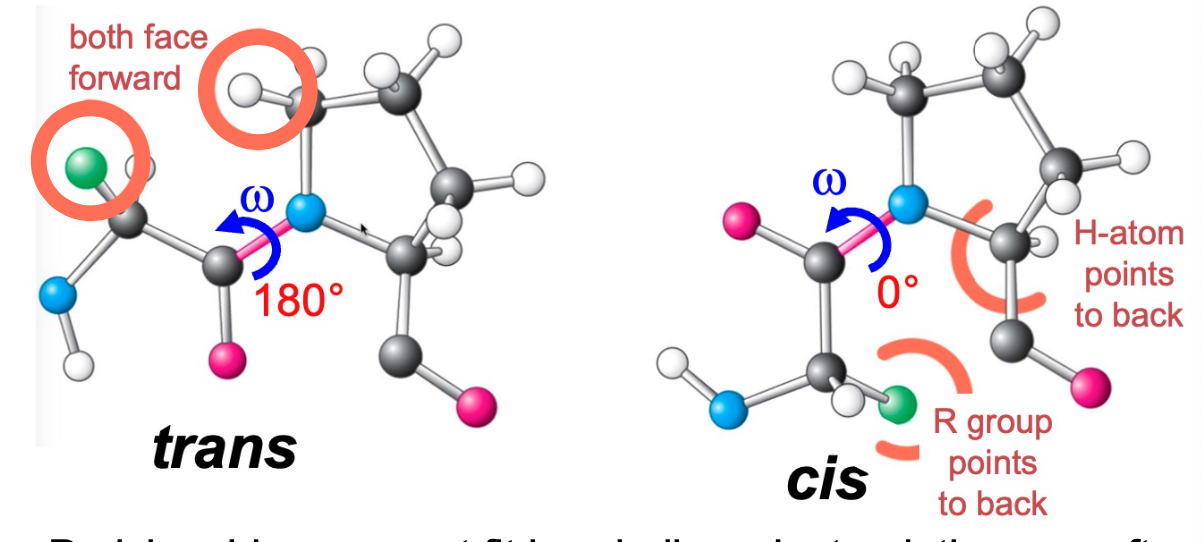
torsion angles of peptide bond
three main ones
psi O=C-C(a)
omega O=C-N (peptide bond)
phi N-C(a)
\*look up symbols to make sure you know them
trans-peptide bonds is observed more than cis-peptide bonds
psi O=C-C(a)
omega O=C-N (peptide bond)
phi N-C(a)
\*look up symbols to make sure you know them
trans-peptide bonds is observed more than cis-peptide bonds
99
New cards
prolyl peptide bonds
cyclic side-chain reduces energetic barrier to adopting cis conformation
most often adopt cis conformation
cannot fit in a=helices, often found at end- helix terminator
most often adopt cis conformation
cannot fit in a=helices, often found at end- helix terminator
100
New cards
secondary structure
helix and sheet structures that arise from the formation of regularly spaced H-bonding between main-chain atoms
Principal secondary structures include:
a-helix
parallel and antiparallel b-pleated sheets
turns- short sequences that connect helices and/or sheets and allow structure that are compact and stable
Principal secondary structures include:
a-helix
parallel and antiparallel b-pleated sheets
turns- short sequences that connect helices and/or sheets and allow structure that are compact and stable