EXAM: chemistry
0.0(0)
Card Sorting
1/360
Last updated 1:05 AM on 12/19/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
361 Terms
1
New cards
7\.4 in
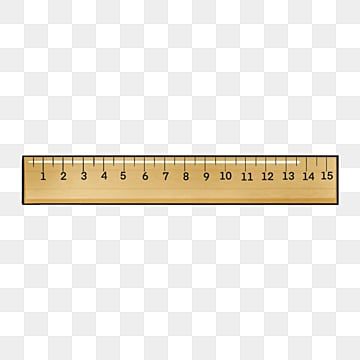
What would be an appropriate measurement using this ruler?
3 in
8\.75 in
9\.236 in
7\.4 in
3 in
8\.75 in
9\.236 in
7\.4 in
2
New cards
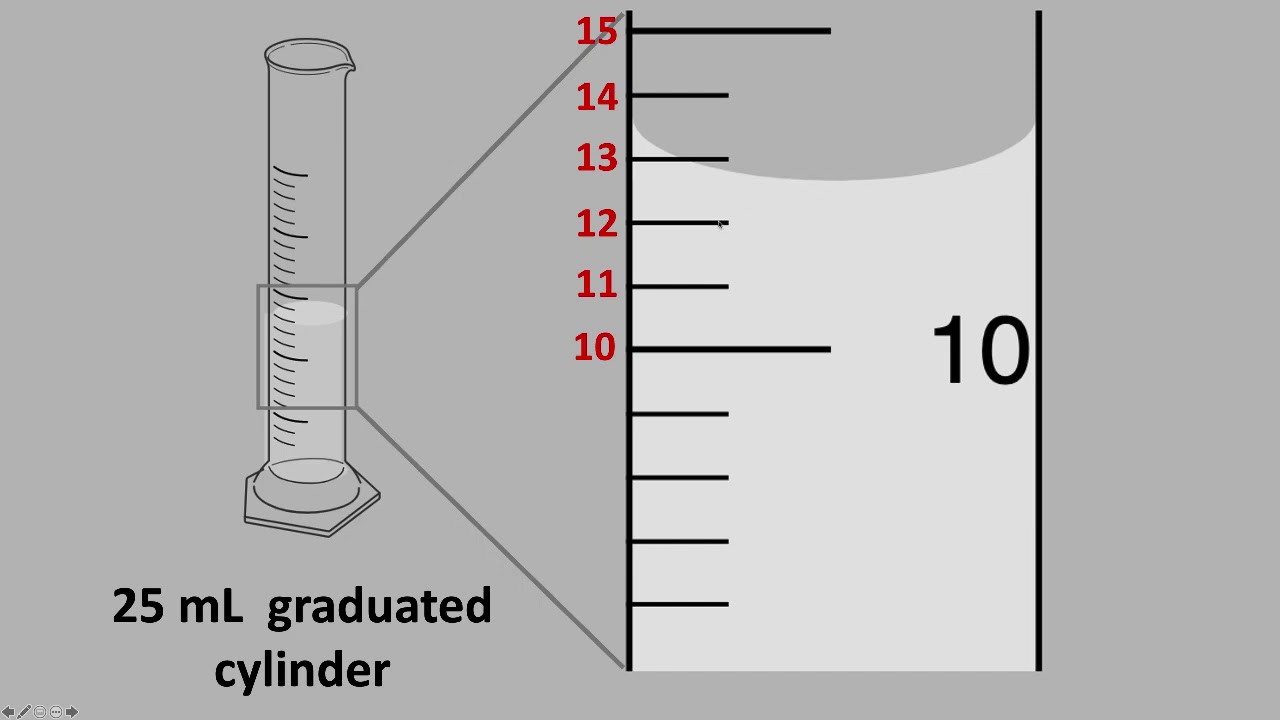
12\.7 mL
What would be an appropriate measurement using this graduated cylinder?
3
New cards
meter (m)
What is the SI unit for length?
4
New cards
kilograms (kg)
What is the SI unit for mass?
5
New cards
kelvin (K)
What is the SI unit for temperature?
6
New cards
seconds (s)
What is the SI unit for time?
7
New cards
SI
Scientists all over the world have agreed on a single measurement system-most other units are derived from these 7 units
8
New cards
derived units
Combinations of SI base units
9
New cards
square meter (m^2)
What is the derived unit for area?
10
New cards
volume (m^3)
What is the derived unit for volume
11
New cards
kilograms per cubic meter (Kg/m^3)
What is the derived unit for density?
12
New cards
kilograms per mole (kg/mol)
What is the derived unit for molar mass?
13
New cards
joule (J)
What is the derived unit for energy?
14
New cards
international system of units
What does SI stand for when discussing units?
15
New cards
accuracy
Refers to the closeness of measurements to the correct or accepted value of the quantity measured
16
New cards
precision
Refers to the closeness of a set of measurements of the same quantity made in the same way
17
New cards
both
Accurate, precise, both, or neither?

18
New cards
precise
Accurate, precise, both, or neither?

19
New cards
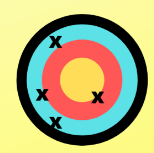
neither
Accurate, precise, both, or neither?
20
New cards
accurate
Accurate, precise, both, or neither?

21
New cards
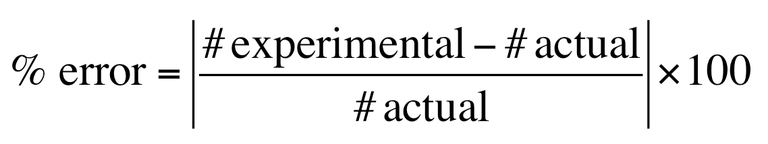
Percent error=
|experimental-accepted|
__________________ x(100)
Accepted
|experimental-accepted|
__________________ x(100)
Accepted
How do you calculate percentage error?
22
New cards
precise but not accurate
3 different people weigh a standard mass of 2.00 g on the same balance. Each person obstains a reading of exactly 4.32 g for the mass. These results imply that the measurement was…
both accurate and precise
accurate but not precise
precise but not accurate
neither accurate nor precise
both accurate and precise
accurate but not precise
precise but not accurate
neither accurate nor precise
23
New cards
true
True or false? All non-zero digits count as significant figures
24
New cards
yes (ex has 3 sf) (other ex has 5 sf)
Are zeros appearing between nonzero digits significant? (ex: 40.7 L) (ex: 87009 km)
25
New cards
no (ex has 5 sf) (other ex has 1 sf)
Are zeros appearing in front of all nonzero digits significant? (ex: 0.095897 m) (ex: 0.0009)
26
New cards
yes (ex has 3 sf)
Do trailing zeros count as significant figures when the number contains a decimal point (ex:1.00\*10^2)
27
New cards
yes (ex has 4 sf) (other ex has 10 sf)
Are zeros at the right end of a number significant? (ex: 85.00) (ex: 9.000000000)
28
New cards
yes (ex has 3 sf)
Do trailing zeros count as significant figures when the number does not contain a decimal point (ex: 100.)
29
New cards
0\.00045
convert 4.5\*10^-4 out of scientific notation
30
New cards
3\.2\*10^6
3200000 in scientific notation
31
New cards
5\.028\*10^-9
0\.000000005028 in scientific notation
32
New cards
91761000
convert 9.1761 × 10^7 out of scientific notation
33
New cards
d=m/v
How do you calculate density?
34
New cards
d=m/v
How do you calculate mass?
35
New cards
d=m/v
How do you calculate volume?
36
New cards
1\.1 g/cm^3
A student measures out 1.5 grams of sodium chloride. The volume is measured to be 1.404 cm3. What is the density?
37
New cards
1\.03 g/mL
An empty graduated cylinder weighs 104.90 grams. A chemist adds 73.5 mL of dilute hydrochloric acid, and it now weighs 180.32 grams. What is the density of the hydrochloric acid?
38
New cards
290 g
A copper cube has a density of 8.96 g/cm^3. The cube is measured to be 3.2 cm on each side. What is the mass?
39
New cards
3\.76%
A student measures the temperature of a liquid with a faulty thermometer, recording a temperature of 314.3 K. Upon repeated measurements by the rest of the class, the temperature is found to be 302.9K. What is the percent error of the first student's measurement?
40
New cards
mole (mol)
What is the SI unit for the amount of something?
41
New cards
physical change
Affects the form of a substance but its identity remains the same
42
New cards
physical change
physical change or chemical change? water freezing into ice
43
New cards
physical change
physical change or chemical change? salt water
44
New cards
chemical change
physical change or chemical change? cooking eggs
45
New cards
chemical change
Affects the chemical identity; the substance changes identities
46
New cards
chemical property
physical property or chemical property? Flammability
47
New cards
chemical property
physical property or chemical property? Combustibility
48
New cards
physical property
physical property or chemical property? Texture
49
New cards
physical property
physical property or chemical property? Solubility
50
New cards
It is a physical property because it changes only the substance’s shape.
Which statement describes why malleability is a physical property?
It is a physical property because it changes the state of the substance.
It is a physical property because it alters only the flammability of the substance.
It is a physical property because it rearranges the substance’s molecules into a new substance.
It is a physical property because it changes only the substance’s shape.
It is a physical property because it changes the state of the substance.
It is a physical property because it alters only the flammability of the substance.
It is a physical property because it rearranges the substance’s molecules into a new substance.
It is a physical property because it changes only the substance’s shape.
51
New cards
physical change
physical change or chemical change? Calcium hydroxide dissolves in water.
52
New cards
chemical change
physical change or chemical change? Baking soda reacts with vinegar, producing bubbles.
53
New cards
chemical change
physical change or chemical change? You add two colorless solutions together and the resulting solid is red.
54
New cards
chemical change
physical change or chemical change? A propane grill is lit, producing a flame.
55
New cards
physical change
physical change or chemical change? Dry ice sublimes from solid to gas.
56
New cards
physical property
A property that can be measured or observed without changing the identity of matter
57
New cards
Length
Color
Density
Mass
Elasticity
Pressure
Volume
luster
Color
Density
Mass
Elasticity
Pressure
Volume
luster
What are the 8 physical properties?
58
New cards
chemical property
A property that can be determined by attempting to change the identity of a substance, but it cannot be determined simply by observing the substance
59
New cards
Production of a new gas (bubbles-not boiling)
Production of heat/light
Production of a precipitate (formation of a new solid)
Change of color
Production of heat/light
Production of a precipitate (formation of a new solid)
Change of color
What are the 4 signs of a chemical reaction?
60
New cards
are rearranged during the change.
When a chemical change occurs, atoms…
are destroyed by the change.
could be created or destroyed.
are rearranged during the change.
are created by the change.
are destroyed by the change.
could be created or destroyed.
are rearranged during the change.
are created by the change.
61
New cards
Dissolving of a solid
Which one is not one of the four signs of a chemical change?
Production of heat and light
Dissolving of a solid
Formation of a gas
Formation of a precipitate
Change in color
Production of heat and light
Dissolving of a solid
Formation of a gas
Formation of a precipitate
Change in color
62
New cards
solid
The state of matter that has a definite shape and volume, and the particles in this state are also closely packed together and orderly.
63
New cards
liquid
The state of matter that has a definite volume and an indefinite shape is, and the particles in this state are in close contact, but randomly arranged and able to flow.
64
New cards
gas
The state of matter that has no definite volume or shape, and the particles in this state are not in contact and are very randomly arranged and move chaotically.
65
New cards
It is a physical property because it changes only the substance’s shape.
Which statement describes why malleability is a physical property?
It is a physical property because it changes the state of the substance.
It is a physical property because it alters only the flammability of the substance.
It is a physical property because it rearranges the substance’s molecules into a new substance.
It is a physical property because it changes only the substance’s shape
It is a physical property because it changes the state of the substance.
It is a physical property because it alters only the flammability of the substance.
It is a physical property because it rearranges the substance’s molecules into a new substance.
It is a physical property because it changes only the substance’s shape
66
New cards
solid
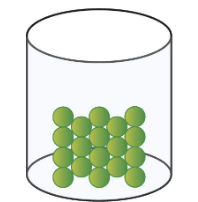
67
New cards
liquid
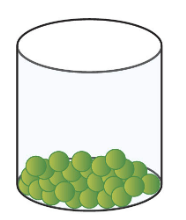
68
New cards
gas
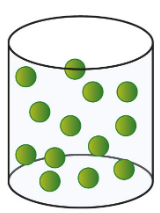
69
New cards
Its atoms are destroyed.
Solid potassium reacts with bromine gas to form solid potassium bromide. Which one of the following is not true of bromine gas?
It undergoes a chemical change.
It is a reactant.
Its atoms are destroyed.
It is an element.
It undergoes a chemical change.
It is a reactant.
Its atoms are destroyed.
It is an element.
70
New cards
extensive property
Depends directly on the amount of substance present
71
New cards
extensive property
extensive property or intensive property? temperature
72
New cards
intensive property
Does not depend on the amount of the substance present
73
New cards
extensive property
extensive property or intensive property? Boiling point
74
New cards
extensive property
extensive property or intensive property? concentration
75
New cards
extensive property
extensive property or intensive property? luster
76
New cards
intensive property
extensive property or intensive property? weight
77
New cards
intensive property
extensive property or intensive property? length
78
New cards
intensive property
extensive property or intensive property? volume
79
New cards
intensive property
extensive property or intensive property? energy
80
New cards
matter
Anything that has mass and takes up space (volume)
81
New cards
mass
A measure of the amount of matter in an object
82
New cards
volume
The amount of space an object takes up
83
New cards
kilogram
What is the common unit for measuring mass?
84
New cards
cubic meters
What is the common unit for measuring volume?
85
New cards
element
A substance that is made up of only one type of atom and can not be broken into a smaller substance by chemical means
86
New cards
compound
A substance that can be broken down into smaller substances by chemical means
87
New cards
pure substance (92 naturally occurring elements)
Matter that always has exactly the same composition
88
New cards
mixture
A combination of two or more substances that are not chemically combined
89
New cards
b
a, b, c, d, e, or f? a mixture of molecules
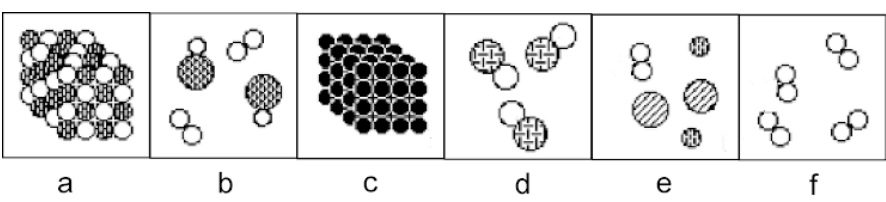
90
New cards
e
a, b, c, d, e, or f? a mixture of elements
91
New cards
a
a, b, c, d, e, or f? a solid compound
92
New cards
c
a, b, c, d, e, or f? a solid element
93
New cards
f
a, b, c, d, e, or f? a gaseous element
94
New cards
d
a, b, c, d, e, or f? a gaseous compound
95
New cards
homogenous mixture
a physical blend of substances that appears the same throughout
96
New cards
solution
What is another name for a homogenous mixture?
97
New cards
heterogeneous mixture
a physical combination of substances that is noticeably different throughout.
98
New cards
homogenous
if a mixture is uniform in composition, so that it's parts will never settle out, it is said to be _____________
99
New cards
intensive
intensive or extensive? Magnetism
100
New cards
intensive
intensive or extensive? Luster