BIOL 2052 - Pleasure and Behaviour
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
pleasure
powerful influence over behaviour and choices we make
consimung pleasurable foods drives us to seek out more of those foods
eating and reward
eating serves an essential role in homeostasis
food intake can also be affected by the pleasurable effects of food
the pleasure of eating food, driven by the “liking” reaction in the brain (also called hedonic circuits)
hedonic feeding driven by the pleasure of consuming food —> component of reward
3 main components make up reward:
liking
wanting (also referred to as incentive salience)
learning - make associations and prediction reward value
motivational circuit can be activated by specific sensory cues like seeing or smelling good tasting food
eating disorders
can induce obesity, bulimia, anorexia
obesity associated with reduced life expectancy and a range of diseases (liver, respiratory conditions, T2D)
homeostatic circuitry
circuit which drives us to seek out food to maintain constant levels
key component is the hypothalamus
recieve signals from the pancreas and brain stem which can change activity
2 types of neurons:
orexigenic: increase appetite
anorexigenic: decrease apetite
reward circuit
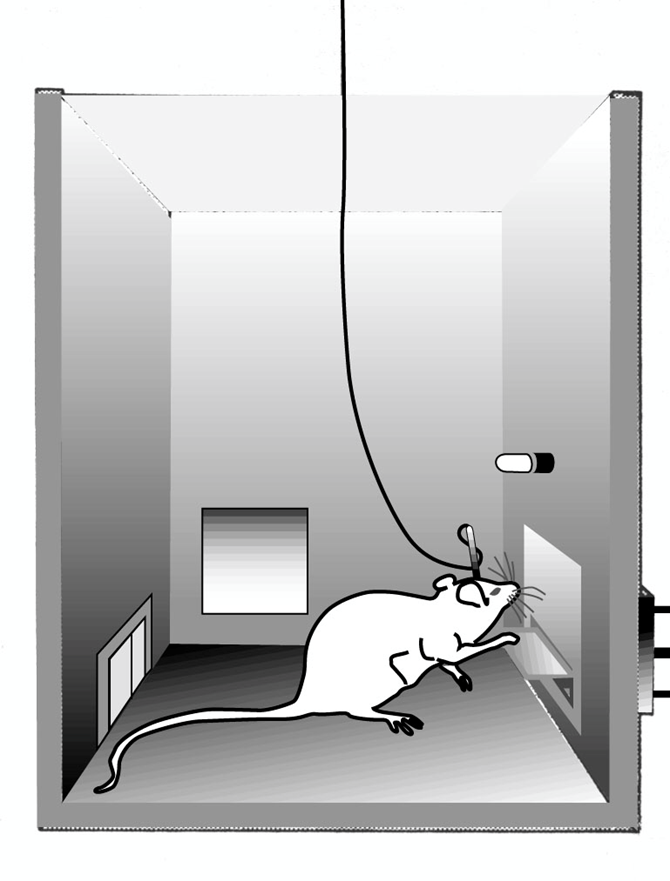
olds and milner: identified reward centre in brain by delivering electrical activity to a certain region of the brain via an electrode
mice self administered stimulation of the brain —> chose to stimulate the reward region of the brain over and over
position of teh electrode was changed and the experiment repeated as a control
circuitry
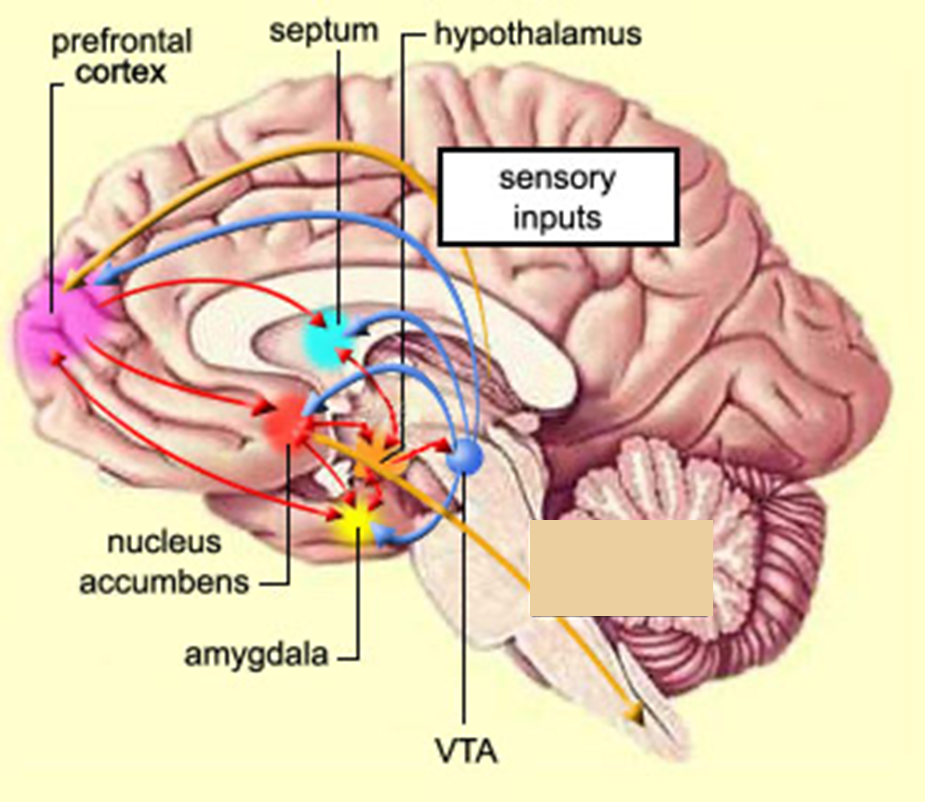
the reward system is the neural network that recieves and evaluates the rewarding properties of stimuli
network consists of multiple interacting neural circuits
robust self stimulation behaviour obtained with electrodes along the medial forebrain bundle
self stimulation observed from electrodes located in several brain areas including:
nucleus accmubens
lateral hypothalamus
ventral tegmental area
cortical structures (insula cortex, orbitofrontal cortex)
key structures of reward circuit
medial forebrain bundle
reward circuit that includes axons that project from the VTA —>Nac (mesolimbic dopamine pathway)
hypothalamus recieves inputs from structures within reward circuits)
hedonic vs neural motivational
conserved facial expressions associated with either liking or adverse reactions
positive facial liking: tongue protrusion
negative facial liking: gaping
tastants are administered to rats, monkeys, babies and the behaviour is quantified by facial liking
signalling pathways
OPIODS
endogenous opiods: enkephalins, dynorphins, endorphins
the opiod receptors: mu, kappa, delta
receptors: all GPCRs
agonists: morphine
antagonists: naloxone
ENDOCANNABANOIDS
lipid molecules such as: anadamide and 2 arachidonoylglycerol
there are 2 subtypes of receptors: CB1 and CB2
receptors are GPCRs
CB1 receptors in CNS
opioid pathways - ate they involved with reward?
morphine increases hedonic pathways
naloxone decreases food intake in rats, especially when sucrose is used
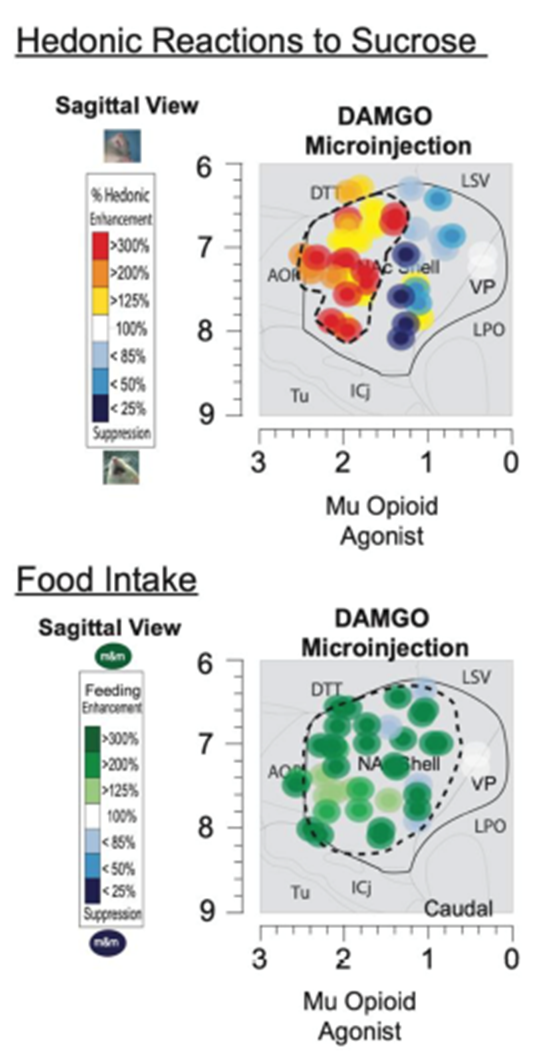
opioid receptors against DAMGO (mu opioid agonist) administered and the measure of facial liking allows us to identify:
role for mu receptors
prescise map for nucleus accumbens
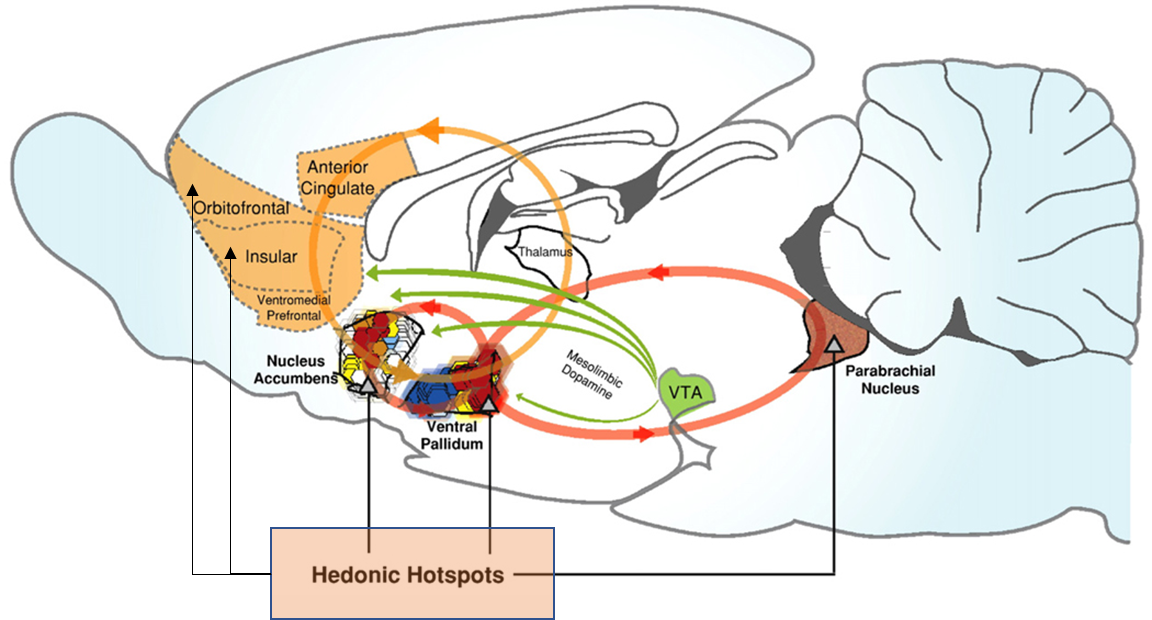
hedonic hotspots (10% of Nacc)
larger region for food intake wanting
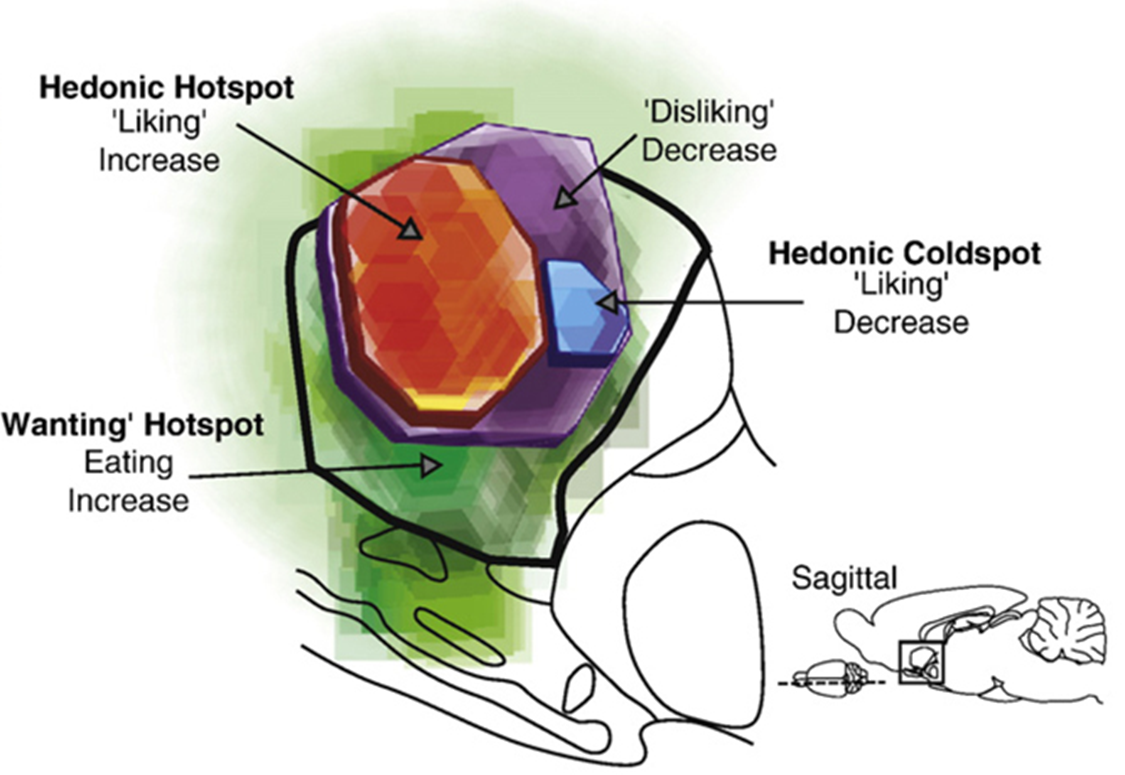
hedonic hotspots of the brain
additional liking structures in addition to the Nacc
mu opiod receptors activation increases in these areas enhanced by hedonic responses to sucrose, by way of increased liking reactions
the different hotspots identified are connected anatonically via neural pathways to create an interconnected circuit for liking
to map the functional connectivity between these hotspots an approach called Fos plume was used
recruitment of hotspots important mechanism for causing increased liking responses
disruption of one hotspot can disrupt liking reactions and the function of the circuit
another experiment done where DANGO injected into one region and naloxone —> activity is recorded
blocking opioid receptors whilst simutantaneously stimulating another hotspot causes the expression of ehanced liking responses
the ventral palladium is an integral hub for liking —> if inhibited/removed it supresses all enhanced liking
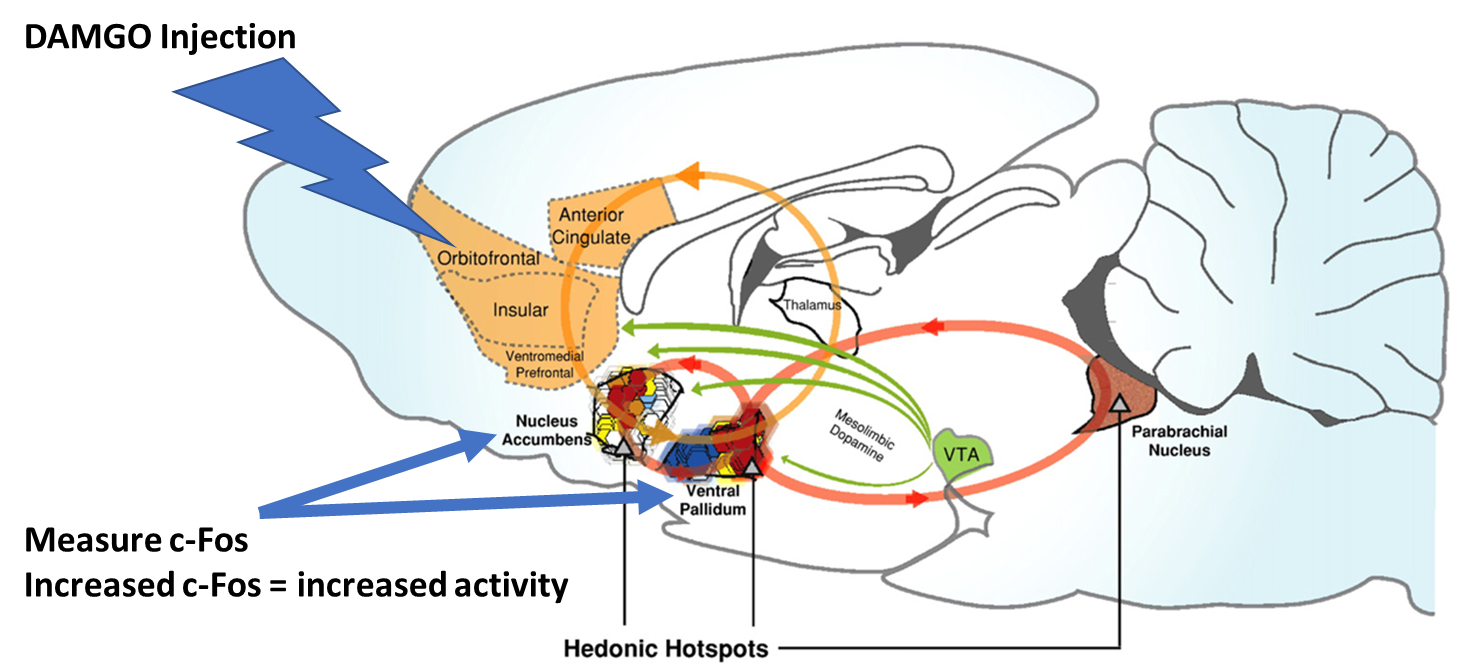
Fos plume
one region of the animals brain is injected with the DAMGO and teh brain is removed
immunhistochemical staining is used to quantify the expression of a protein called cFos in regions of the brain outside the injection site
cFos is a protein that is expressed when neurons are activated and so increased expression of this protein provides a readout for when neurons are being activated by the drug injected
endocannabanoid pathway
anecdotal evidence: active ingredient for cannabis is 9 tetrahydrocannabanoidal increases food intake, particularly sugary food
targeted injection of anandamide into Nacc increases facial liking expression to sucrose
therefore, theres an endocannabanoid hotspot in the Nacc that overlaps with the opiod hotspot
CB1 receptors and mu receptors have been identified as co localised and can function together to coordinate release of GABA/glycine
motivational pathway
dopamine is the key neurotransmitter for reward
hypothesis testing used the facial expression paradigm compared with the neurochemical lesions in the brain to reduce dopamine signalling
depletion of dopamine did not affect orofacial expression of liking response to sweetness
but reducing dopamine caused rats to no longer seek out or consume food
so: dopamine more involved with motivation component rather than pleasure
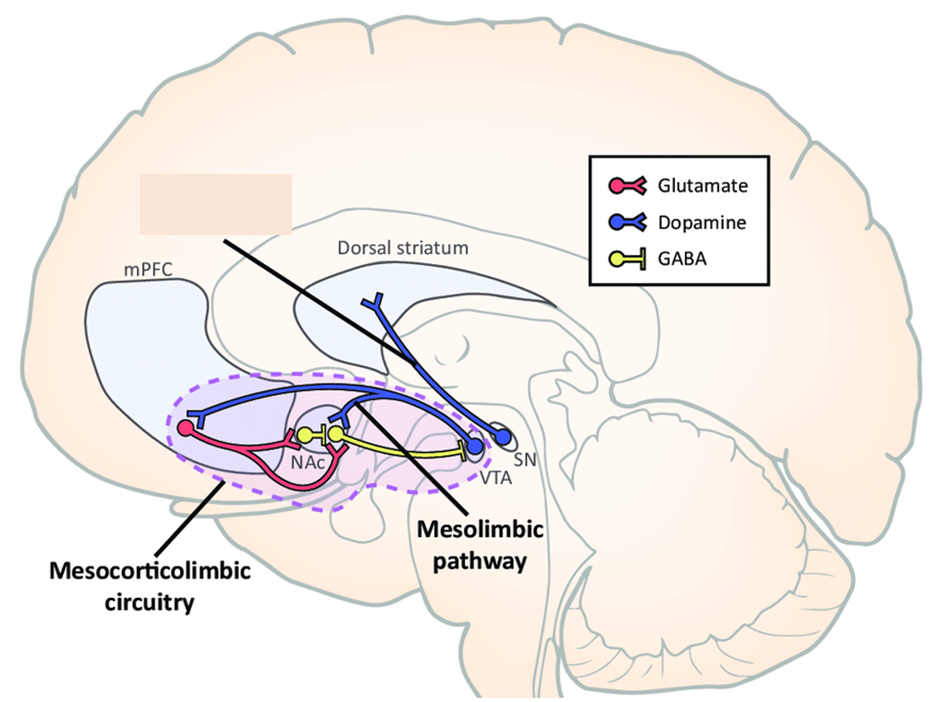
further evidence
G OHDA is able to remove the effect of dopaminergic neurons —> doesnt affect pleasure but does surpass wanting
mice with increased dopamine have increased wanting for sweet food but no increase in liking
individuals with low dopamine (parkinsons) show no difference in pleasure
cross talk of reward homeostasis
orexin is a signalling molecule for cross talk between reward and homeostatic signalling
cross talk occurs at the hypothalamus
evidence for this:
orexic neurons project from the lateral hypothalamic neurons to structures of the reward system
orexin microinjections in hedonic hotspots enhances liking reactions to sucrose
eating disorders
some individuals have the wanting circuitry which exceeds the liking circuitry causing them to be vulnerable to food wanting triggered by associated sensory cues
this leads to overeating
incentive sensation caused by hyperactivity of the dopaminergic pathways in the reward circuitry
more intense triggering of wanting pathways in reponse to palatable food cues and associated with visual image
future direction: tracking changes in brain function and activity using neuroimaging to understand changes that take place before/after obesity develops