[MEMORIZE] Elements in the Periodic Table (Groups, First 30 elements and some).
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
The Periodic Table
See Image
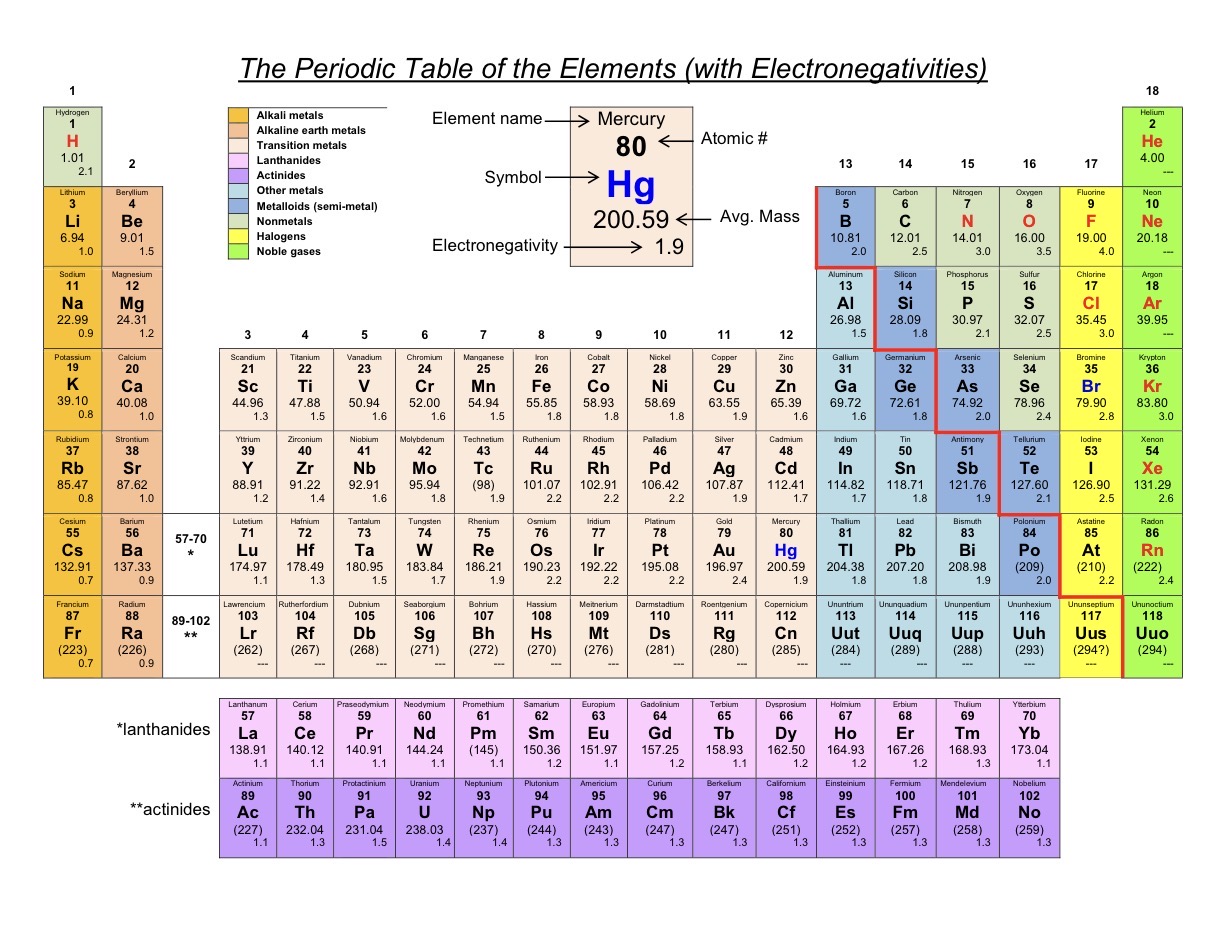
Red line staircase on periodic table
Portrays the metalloids (generally considered nonmetals when it comes to forming anions or cations).
Elements to the right of metalloids are
Nonmetals.
Elements to the left of the metalloids are…
Metals.
Main Group Elements
Groups 1,2 3-18.
Group 1
Alkali metals. Form +1 charge.
Group 2
Alkaline Earth Metals, form +2 charge.
Transition Metal
Groups 3-12. (These form cations as they are considered metals).
Lanthanides & Actinides
Inner Transition metals.
Group 13
+3 charge.
Group 15
-3 charge. Pnictogens.
Group 16
-2 charge. Chalcogens.
Group 17
-1, halogens
Group 18
Noble gases, don’t form ions.
H
Hydrogen.
He
Helium.
Li
Lithium.
Be
Beryllium.
B
Boron.
C
Carbon
N
Nitrogen.
O
Oxygen.
F
Flourine.
Ne
Neon.
Na
Sodium.
Mg
Magnesium.
Al
Aluminum.
Si
Silicon.
P
Phosphorus.
S
Sulfur.
Cl
Chlorine.
Ar
Argon.
K
Potassium.
Ca
Calcium.
Sc
Scandium.
Ti
Titanium.
V
Vanadium.
Cr
Chromium.
Mn
Manganese.
Fe
Iron. (Could be +2 or +3 charge).
Co
Cobalt.
Ni
Nickel.
Cu
Copper.
Zn
Zinc.
Se
Selenium.
Br
Bromine.
Kr
Krypton.
Ag
Silver.
I
Iodine.
Xe
Xenon.
Pt
Platinum.
Au
Gold.
Hg
Mercury.
Pb
Lead. (Can be +2 or +4 charge).
Rn
Radon.