CFCs and chloroalkanes
1/7
Earn XP
Description and Tags
free rad sub :( (i don't know if we need to know the whole free rad sub for CFCs)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
give the molecular formula for ozone:
O3
why is UV radiation dangerous?
damages DNA, causing skin cancers
why is ozone significant in the environment?
absorbs UV radiation
what is a CFC?
compound containing only Cl, F and C atoms
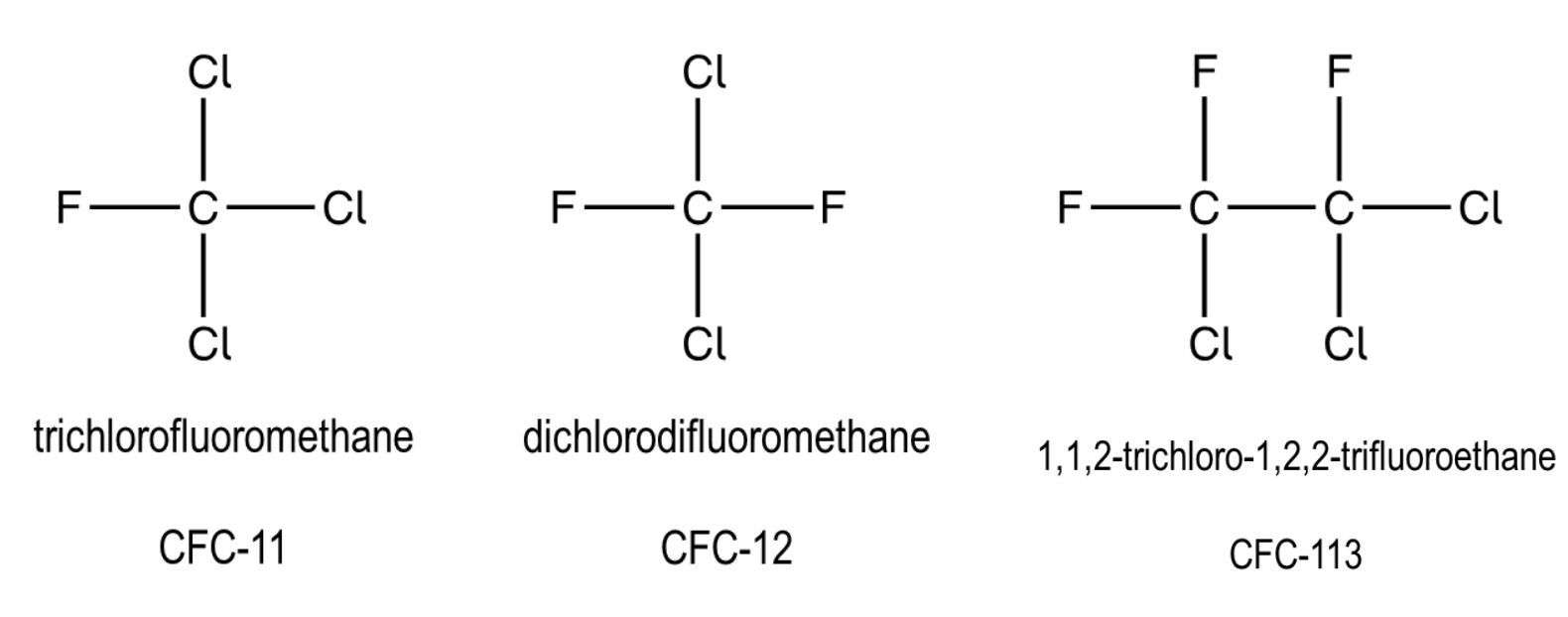
what were CFCs used as?
refridgerants
solvents
why are CFCs dangerous?
Cl radicals formed in upper atmosphere through initiation when UV radiation causes C-Cl bonds in CFCs to break
these Cl radicals catalyse the decomposition of ozone and contribute to the hole in the ozone layer
how was the issue of CFCs resolved?
research provided evidence for CFCs to be banned as solvents and refridgerants
alternative Cl free compounds developed
how do Cl radicals catalyse the decomposition of ozone? which step is this? provide the equations
propagation:
Cl . + O3 → ClO . + O2
ClO . + O3 → Cl . + 2O2
overall: 2O3 → 3O2