Electronic Configuration
0.0(0)
Card Sorting
1/3
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
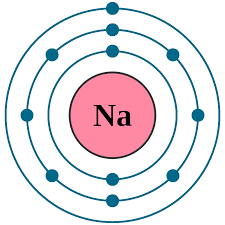
Period
The amount of shells + How far down horizontally on the periodic table
NA (Sodium) in Period 3 and has three shells.
2
New cards

Atomic Number
The number of protons in the nucleus of an atom (top number) + total number of electrons in all shells
Na = 11
3
New cards
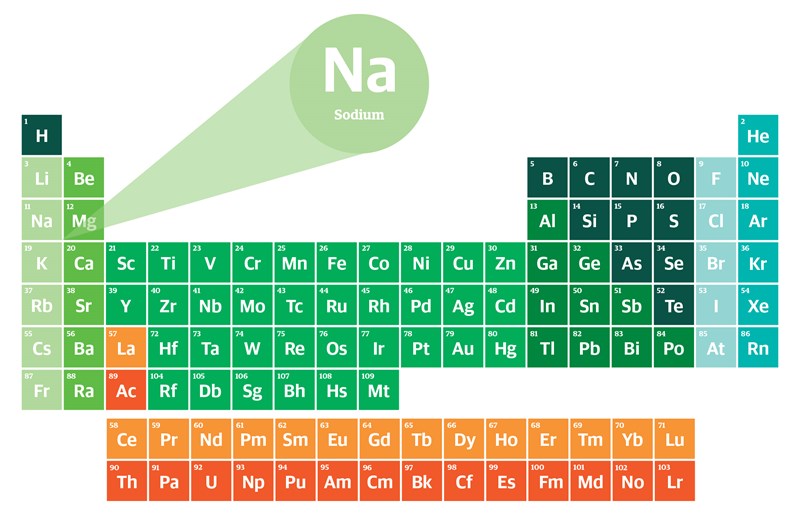
Group Number
The column number (vertical) on the periodic table + number of electrons in only the outershell
Na Group 1 = 1 electronic in the outershell
4
New cards
What is electronic configuration?
The arrangement of electrons into shells.