Rate of reaction
0.0(0)
Card Sorting
1/25
Earn XP
Description and Tags
external pratice
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
1
New cards
catalyst definition
provides an alternative lower activation energy pathway for the reaction to occur without being consumed in the process.
2
New cards
catalyst expl
more collisions occur with greater than the Ea - so a greater proportion of successful collisions/s
3
New cards
Catalyst diagram
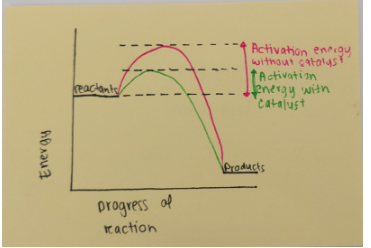
4
New cards
Concentration
more particles/mL
more collisions/s
5
New cards
Surface Area
more particles exposed
more collisions/sand increased reaction rates.
6
New cards
ROR - Rate of reaction comparison statement
there was the same AMOUNT of reactants in both reactions therefore the same AMOUNT of products are formed.
7
New cards
Temp
Faster —> more collisions/s
more kinetic energy
more collisions occur with greater than Ea
more successful collisions/s
8
New cards
9
New cards
10
New cards
11
New cards
12
New cards
13
New cards
14
New cards
15
New cards
16
New cards
17
New cards
18
New cards
19
New cards
20
New cards
21
New cards
22
New cards
23
New cards
24
New cards
25
New cards
26
New cards