CHEM250 Thermodynamics and Aldols
0.0(0)
Card Sorting
1/14
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
1
New cards
Reaction profile
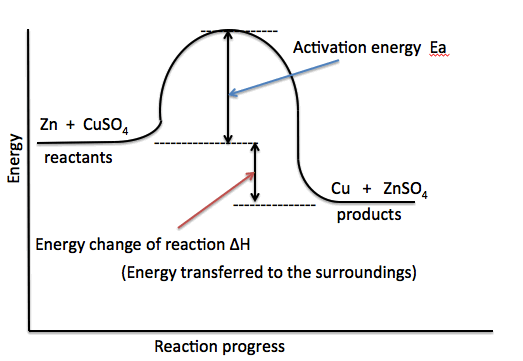
2
New cards
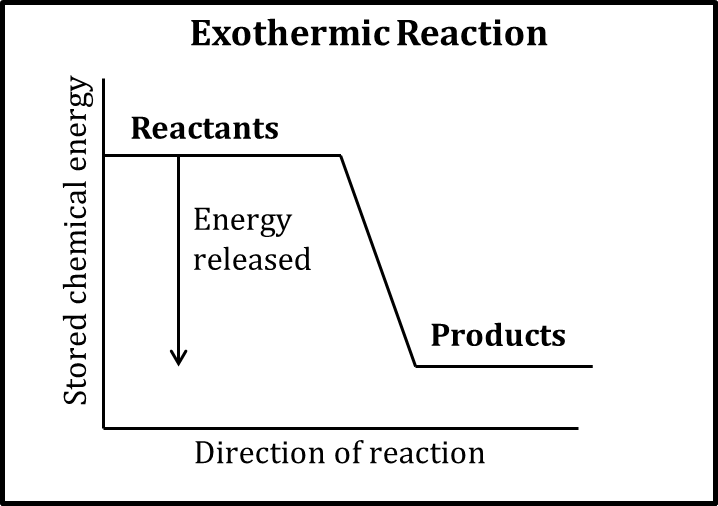
Exothermic (enthalpy)
* Energy released (favored)
* Making bonds
* -ΔH
* Making bonds
* -ΔH
3
New cards
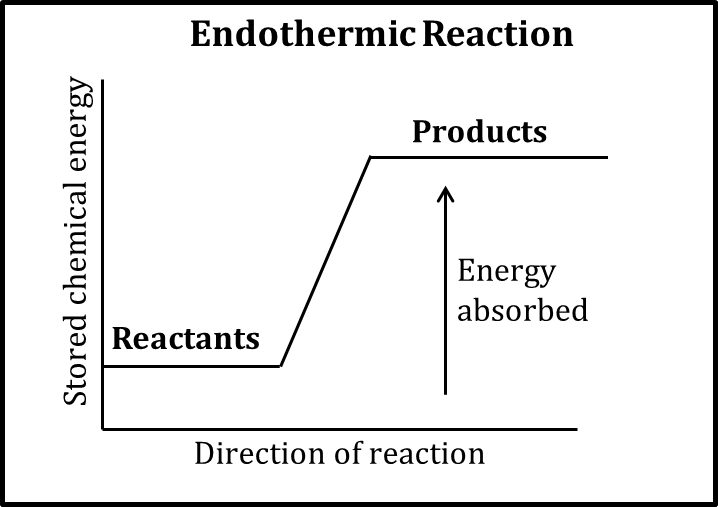
Endothermic (enthalpy)
* Energy absorbed (disfavored)
* Breaking bonds
* +ΔH
* Breaking bonds
* +ΔH
4
New cards
Entropy
* -ΔS: degrees freedom decrease (disfavored)
* +ΔS: degrees freedom increase (favored)
* +ΔS: degrees freedom increase (favored)
5
New cards
Gibbs Free Energy
ΔG = ΔH - TΔS
\
* -ΔG: favored reaction, exergonic
* +ΔG: disfavored reaction, endergonic
\
* -ΔG: favored reaction, exergonic
* +ΔG: disfavored reaction, endergonic
6
New cards
If a reaction is **reactant** favored, a _____ base was used.
weak
7
New cards
If a reaction is **product** favored, a _____ base was used.
strong
8
New cards
Using a weak base results in how much enolate anion being formed?
little
9
New cards
Using a strong base results in how much enolate anion being formed?
lots
10
New cards
Le Chatelier’s Principle
when a system experiences a disturbance (such as concentration, temperature, or pressure changes), it will respond to restore the ratio of products to reactions (Keq) to the equilibrium set value.
11
New cards
Reversible reaction
* -20 kJ/mol < ΔGrxn < 20 kJ/mol
* 10^-3 < keq < 10^3
* 10^-3 < keq < 10^3
12
New cards
Irreversible, product favored reaction
* ΔGrxn < 20 kJ/mol
* keq > 10^3
* keq > 10^3
13
New cards
Irreversible, reactant favored reaction
* ΔGrxn > 20 kJ/mol
* keq < 10^-3
* keq < 10^-3
14
New cards
List two ways that you can push a reaction with a small ΔG to products.
1. Add more reactants
2. Remove the products as the reaction is occurring
15
New cards
List the bases that will irreversibly and completely convert an aldehyde or ketone into an enolate.
* LDA
* NaH
* NaH