Chemistry- Physical chem fundamental particles
1/4
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
John Dalton atomic model
Atoms were spherical indivisible units of matter
JJ Thompson Plum pudding model
He discovered atoms were divisible and said the atom was a positively charged cloud with negatively charged electrons embedded in it
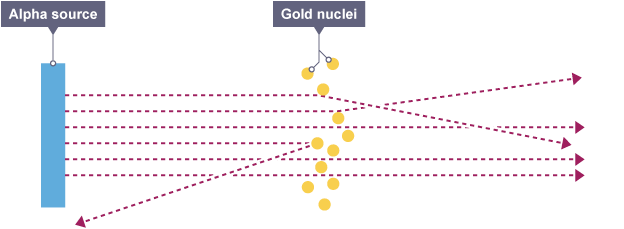
Ernest Rutherford nuclear model
Most of the alpha particles fired went straight through which meant most of the atom is empty space.
Some particles deflected suggesting most of the atom’s mass was positive and concentrated at the center, as like charges repel.
Some particles bounced back, suggesting most of the mass was concentrated in a tiny volume at the center.
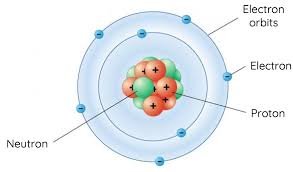
Bohr’s atomic model
The Bohr model (named after Danish physicist Niels Bohr) of an atom has a small, positively charged central nucleus and electrons orbiting in at specific fixed distances from the nucleus. Electrons are not allowed to orbit in the space between these specific fixed orbits.
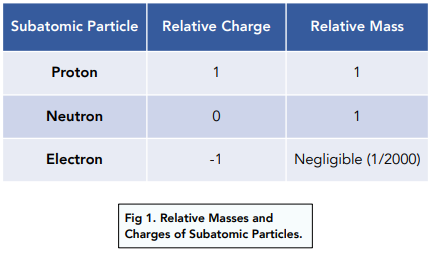
Mass and charges of sub atomic particles
Proton 1 and +ve
Neutron 1/2000 and NA
Electron 1 and -ve