Chapter 8: Gas Laws
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
Pressure
collision of gas molecules with wall of container
Temperature
related to average speed of gas molecules
A Closed-End Manometer
P = d × g × h
Boyle’s Law: Pressure and Volume Equation
P1V1 = P2V2
Charles’s Law Equation
V1/T1 = V2/T2
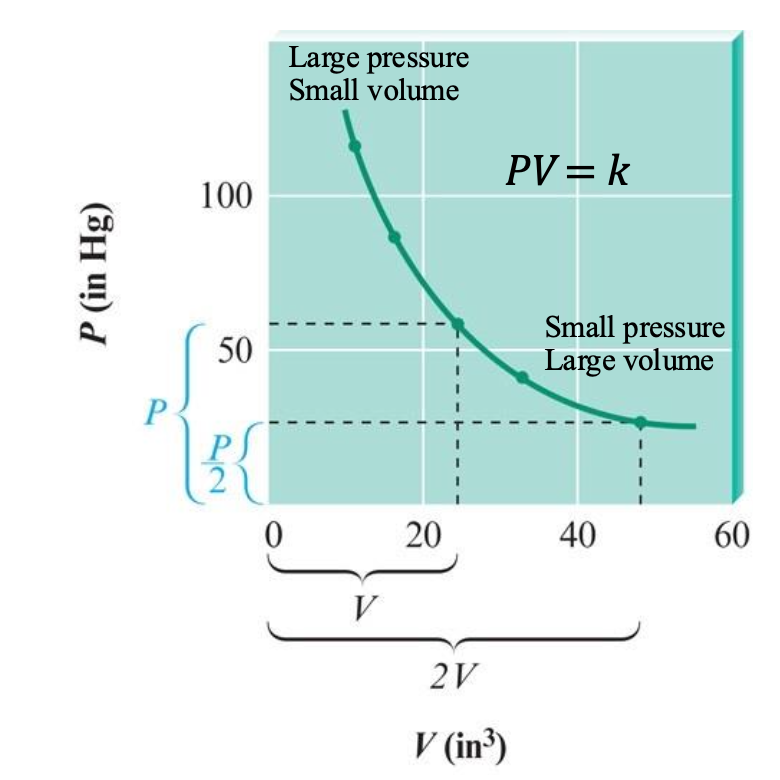
P versus V
obtain a curved line called a
hyperbola
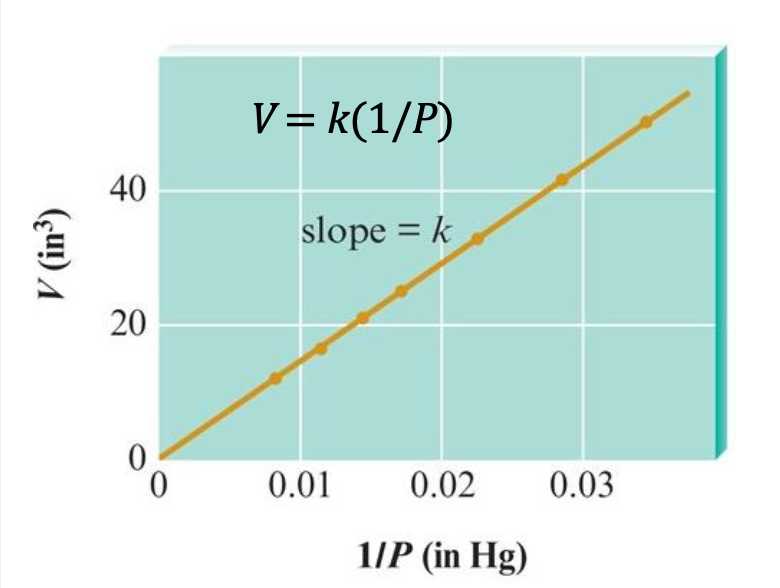
V versus 1/P
we obtain a straight line
Gas Law of Boyle
Volume increases, pressure decreases
Gas Law of Charles
Temperature increases, volume increases
Charles’s Law Graph - By plotting V versus T (℃)
we obtain straight lines
Gas Law of Avogadro
Moles increase, volume increases
Gas Law of Avogadro Equation
V1/n1 = V2/n2
The Ideal Gas Law Equation
PV=nRT
Combined Gas Law Equation
P1V1/T1 = P2V2/T2
Molar Mass of a Gas Equation
dRT/P
All gases, regardless of their molecular mass, have the same
average kinetic energy at the same temperature.
Mole Fraction of a Gas
the ratio of the number of moles of a given
component in a mixture to the total number of moles in the
mixture.
❖ Represented by chi ( χ):
n1 n1
χ1 = –––– = –––––––––––––––
ntotal n1 + n2 + n3 + …
Dalton’s Law of Partial Pressures
Ptotal = P1 + P2 + P3 + …
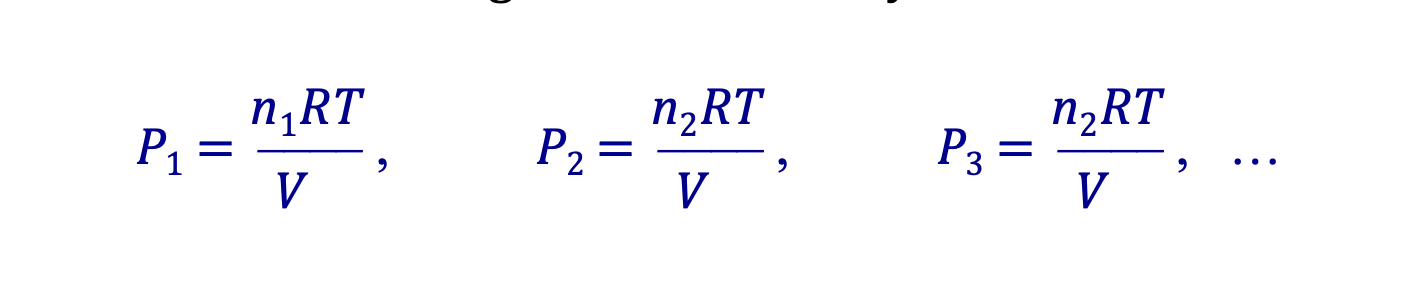
Diffusion
refers to the process of particles (atoms, molecules, or ions)
moving from an area of higher concentration to an area of lower
concentration.
Effusion
is the process in which a
gas escapes from its container
through a tiny hole, or orifice into
a vacuum.
Real gases deviate from the ideal gas law
for two main reasons:
1.) Intermolecular forces of attraction cause the
measured pressure of a real gas to be less than
expected.
2.) When molecules are close together, the volume of
the molecules themselves becomes a significant
fraction of the total volume of a gas.
Ideal Gas - No volume and no intermolecular attractions
High Pressure and Low Temperatures cause there to be deviations
Postulates of the Kinetic Molecular Theory
1.) The particles are so small compared to the distances between
them that the volume of the individual particle can be
assumed to be negligible or zero.
2.) The particles are in constant motion.
The collisions of the particles with the walls of the container
are the cause of the pressure exerted by the gas.
3.) The particles are assumed to exert no forces on each other.
They are assumed neither to attract nor to repel each other.
4.) The average kinetic energy of a collection of gas particles is
directly proportional to the gas temperature.
All gases, regardless of their molecular mass, have the same
average kinetic energy at the same temperature.
Ozone formation
O₂ → O₃ via UV light
Ozone depletion
: CFCs release Cl radicals
Greenhouse gases
(CO₂, CH₄) trap heat
Environmental Impact
Smog, acid rain, global warming
Strategies: reduce emissions, catalytic converters, renewable energy
Real gases deviate from the ideal gas law: Reason #1- forces of attraction cause the
measured
Intermolecular forces of attraction cause the
measured
pressure of a real gas to be less than
expected.
Real gases deviate from the ideal gas law: Reason #2 - molecules are close together, the volume of
the molecules themselves
becomes a significant fraction of the total volume of a gas.
Van der Waals Equation of State for Gases
Ideal behavior of real
gases at high temperatures can be explained with this equation
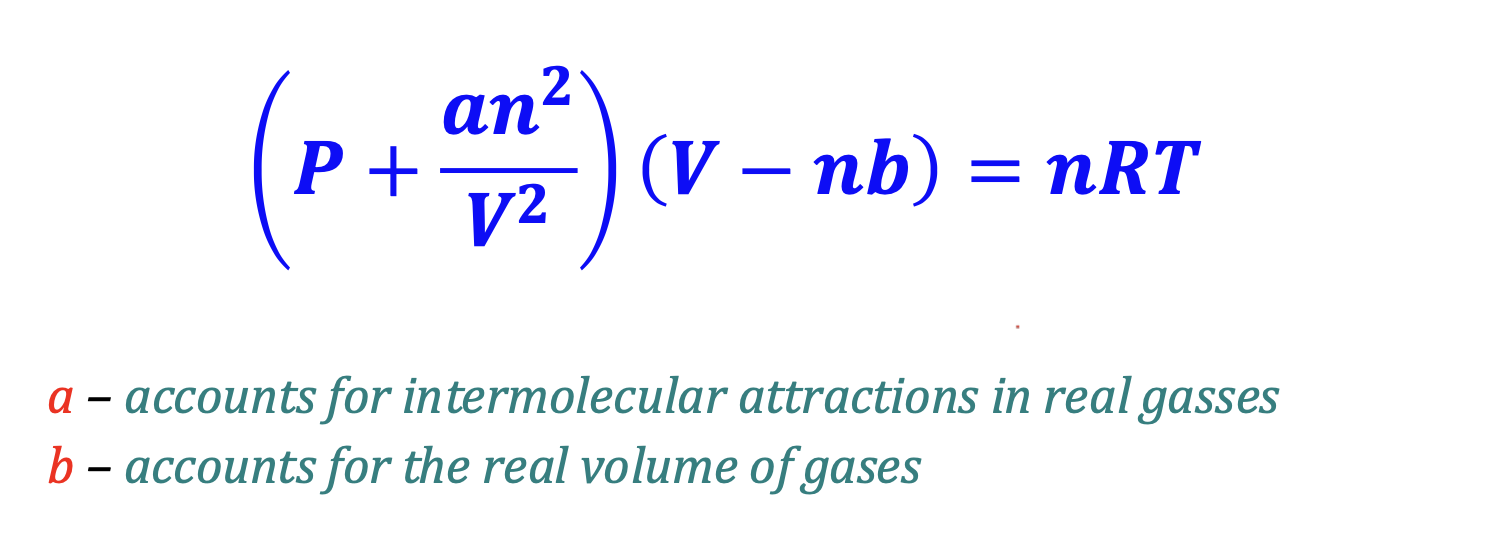
Number of Moles INCREASE
Average kinetic energy remains the same
Average velocity remains the same
Collision Frequency increases
Pressure increases
Average kinetic energy remains the same
Average velocity remains the same
Collision Frequency increases
When Temp increases
Average KE increases
Average V increases
Collision frequency increases
Volume Decreases
KE remains the same
Velocity remains the same
Collision frequency increases