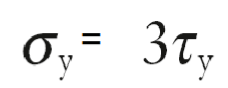Strengthening Mechanisms
1/39
Earn XP
Description and Tags
MEE1004: Mechanics of Matei
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
Hardness Tests
A localised non destructive measurement
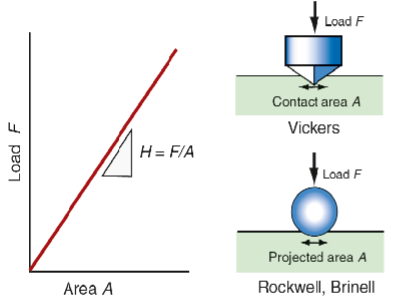
Hardness test equation
F = test force
A = Surface area of indentation
σy = yield strength

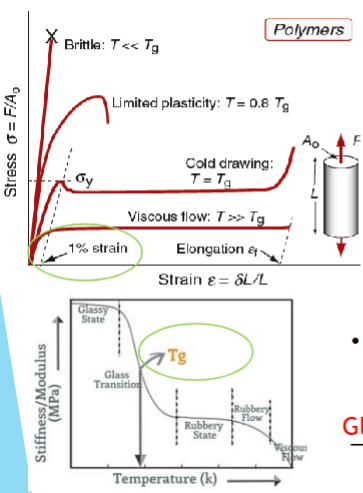
Strength of polymers
σy: stress at which the stress-strain curve becomes markedly non-linear. Typically around a strain value of 1%.
Behaviour beyond the yield depends on polymer’s glass transition temperature.
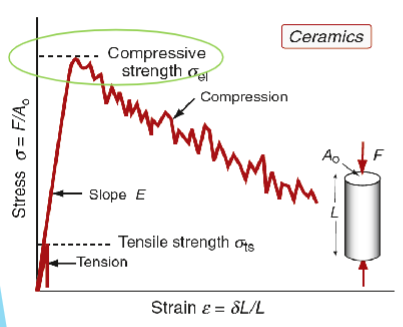
Strength of Ceramics
Yield strength never reached before material fractures.
Measurable compressive strength in used in practice elastic limit (σel)
Strength of a material
The force necessary to break inter-atomic bonds.
The atomic bond is broken when it is stretched to 10% of its original length.
Ideal strength equation
σideal: maximum possible stress a perfect material can withstand
E: Young’s modulus of the material
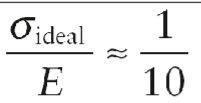
Why no material achieves ideal strength value
Defects that exist within the material, such as interior or surface cracks.
Defects in metal and ceramics
Vacancies, solute atoms, grain boundaries, dislocations
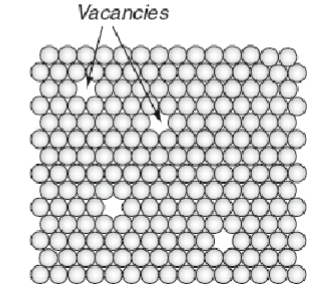
Vacanies
A site at which an atom is missing
Plays a role in diffusion, creep and sintering
They do not influence the strength of materials
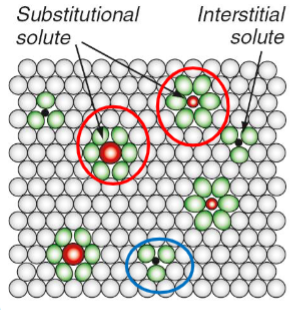
Solute atoms
Substantial solid solution: atoms replacing the host atoms.
Interstitial solid solution: Squeeze into spaces or “interstices” between the host atoms.
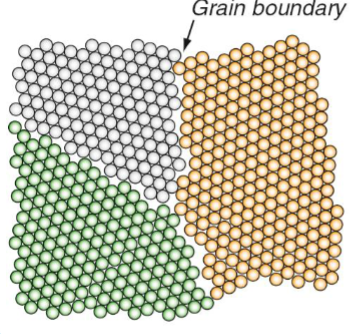
Grain boundaries
Dislocations pile up at grain boundary, creating the stress needed in the next grain to nucleate the matching dislocations.
Dislocations
An extra half-plane of atoms in the crystal which do not bond with other atoms underneath to form a continuous plane.
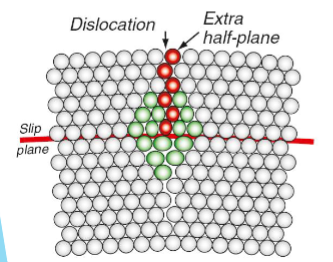
Dislocation movement
The main source of plastic deformation in crystalline materials,
Dislocation movement along shear plances
Plastic deformation of a crystalline material corresponds to the motion of large numbers of dislocations.
Multiple slip displacements traverse crystal = deformation at a macroscopic level.
Dislocation prefer to move on slip planes and along slip directions.
Slip plane / direction with highest atomic density is preferred.

Manipulating Strength of metals
Strengthening crystallie materials by making it harder for disolations to move (i.e. enhancing the shear yield strength).
Dislocation pinning
A mechanism that halts a dislocation’s movement, requiring a greater amount of force to be applied to overcome the barrier.
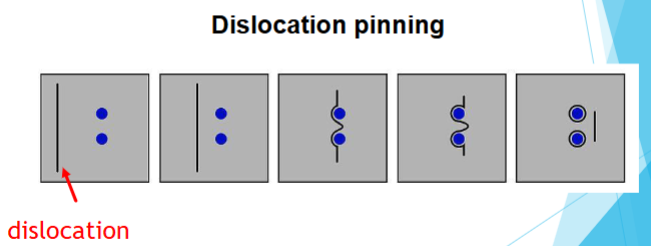
How metals are strengthened
Solution hardening (alloying),
Dispersion and precipitate strengthening,
Work hardenung / strain hardening,
Grain boundary hardening
Solution hardening (alloying)
Strengthening of metals by alloying - deliberate additions of impurities (solutes).
Alloying process strengthens the metal by adding other elements that disperse atomically into the host lattice.
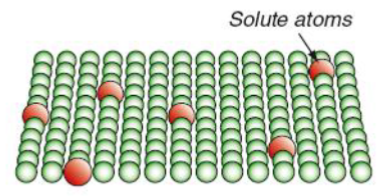
Ways to increase the effectiveness of alloying
Increase amount of alloying element, increases solid solution strengthening.
Increase the size difference between the alloying elemets and the host material, the harder it is for dislocations to move.
Increase amount of alloying elements increase soid solution strengthening.
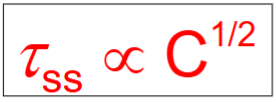
Calculating the contribution of solid solution to the shear stress required to move the dislocation
C = concentration of solute
τss = additional shear stress required due to solid solution strengthening
Dispersion
Disperse small, strong particles of higher melting point into a liquid metal, the particles trapped when metal cast into shape (e.g. metal matrix composite, Al-SiC).
Precipitation
A heat treatment process that produces uniformly dispersed particles within a metal’s grain structure.
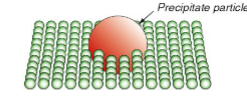
Precipitation hardening
Makes it more difficult for the dislocation to shear and travel across them.
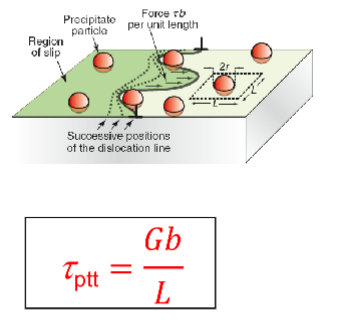
Shear stress required to move dislocation due to disperion and precipitate strengthening
τppt = shear stress required to move dislocation
L = spacing of particles
G = alloy shear modulus
b = magnitude of slip factor
Work hardenigng
Caused by accumulation of dislocation generated by plastic deformation.
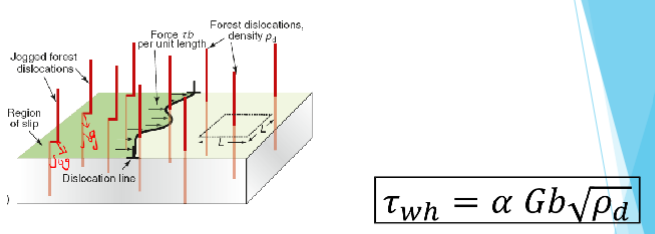
Work hardening and shear stress
Contribution of work hardening to the shear stress required to move the dislocation
Work hardening and shear stress equation
τwh: shear stress required to move the dislocation
α: constant
G: shear modulus
b: length of jogs
ρd: density of dislocation
Dislocation density
The length of dislocation line per unit volume (m/m3).
Dislocation density equation
Dislocation density: total dislocation length/unit volume
Another word for work hardening
Strain hardening
Examples processes for strengthen metals through cold working (work hardening)
Rolling, forging, extrusion and drawing.
Grain boundary hardening
Method of strengthening materials by changing their average crystallite (grain) size.
Effects of grain boundaries on the shear strength required for a dislocation to move
τgb: shear stress
D : grain size (typically 10 - 100 μm)

Can strengthening mechanisms be used in combination
Yes
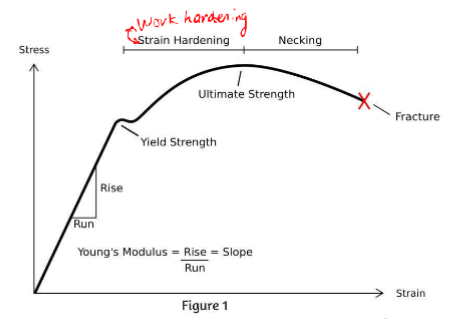
Strength and ductilility
An increase in strength will lower the ductility
(lower elongation to fracture, i.e. more brittle)
Approximation of shear yield strength based on strengthening mechanisms.
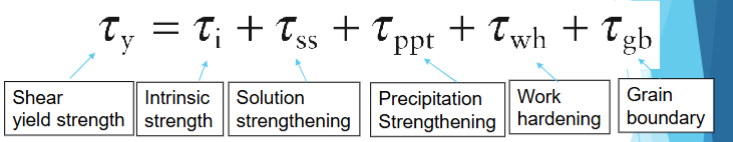
How to link tensile yield strength to the shear yield strength
Θ is the angle between shear plane normal and the tensile loading direction
Dislocations will move on the slip plane on which this shear stress is the greatest
Maximum shear stress lies at an angle of 45 when τ = σ/2, i.e. σy ∝ τmax

Strengthening polymers and relative slippage
For strengthening polymers, relative slippage of polymer chain must be considered.
Impeding the slippage of molecular chains can be done through blending, drawing, cross-linking, and by reinforcement with particles, fibres and fabrics.
Crystal resistance to dislocation
Crystals resist the motion of dislocations with a friction-like resistance (lattice resistance) f per unit length.
Dislocation move if τ > f/b
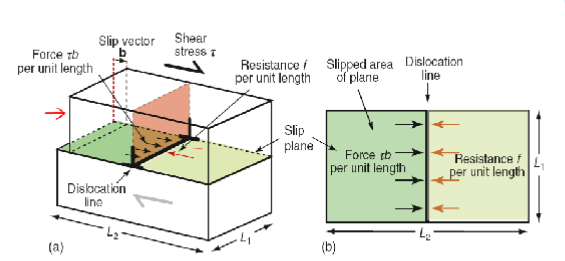
Calculating tensile stress required to cause yielding
Tensile stress required to cause yielding is 3x the shear strength of a single crystal.