PHM 431 Exam 2
1/124
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
125 Terms
What are the combinations of GABAa receptor's subunits?
a1,b2,y2 (60%)
a2,b3,y2 (20%)
What subunits do Barbiturates and alcohol bind to on the GABAa receptors?
On the beta subunits
What is the potency of alcohol like?
Alcohol has a very poor potency. There are 14 grams of EtOH per drink. A blood alcohol level of 0.1% means that there is 100 mg of alcohol per 100 mL of blood.
What is Naltrexone treatment for alcoholism?
This is an opioid receptor antagonist. It is used for extreme cases because it blocks your endogenous opioid receptors completely. It blocks the mu opioid receptors in the VTA, which causes the release of GABA thus inhibiting the release of dopamine in the prefrontal cortex and nucleus accumbens (both D1 receptors). Essentially it stops the reward circuit and does not allow alcohol to give the same euphoric effects.
Acomprosate for treatment of alcoholism?
It is a long-term treatment of withdrawal, it prevents withdrawal symptoms like alcohol craving. It has no effect on the reward pathway.
What are the endpoints of Phase 1 Clinical Trials?
Safety and tolerance; they use healthy human volunteers
What happens Phase 2 Clinical Trials?
Several hundred patients with the target disease. It can involve multiple institutions. It is about proving efficacy.
What happens during a phase 3 clinical trial?
Must show clinical efficacy; many drugs die at this point even though the company has invested millions of dollars.
Alcohol is classified as a:
GABA A receptor agonist; it increases intracellular Cl- which causes the cell to hyperpolarize. Overall, it depresses the CNS
Alcohol Pharmacokinetics: Absorption
- Absorbed through the portal blood from the stomach and intestines (portal veins carry alcohol blood to the liver)
- slow absorption into the system
- long latency
- large first-pass effect.
Alcohol Pharmacokinetics: Distribution
- Small, uncharged molecule
- Easily gets across the blood-brain barrier
What are the enzymes that process alcohol?
In the liver: make acetaldehyde
- Alcohol Dehydrogenase
- P450
Then acetaldehyde is processed by Aldehyde Dehydrogenase to make Acetic Acid
What is the process of alcoholic liver disease?
1. Alcohol Ingestion
2. Fatty Liver
3. Alcohol Hepatitis
4. Cirrhosis
What does disulfiram do?
inhibits aldehyde dehydrogenase, which inhibits the production of acetic acid. It causes hangover symptoms and discourages chronic users from drinking.
What is the rate of elimination for alcohol?
It follows zero-order elimination kinetics, which means that the rate of metabolism is set.
What does alcohol do to the brain on a molecular level?
It is a GABAa receptor agonist, which allows in influx of Cl- ions. This causes an over-hyperpolarization of the cell, which causes the drunk feeling.
What brain regions does alcohol directly affect?
It targets the projection neurons in the amygdala and hyperpolarizes them. This causes the inhibition of neurons in the cortex and hypothalamus and the decreased release of serotonin, norepinephrine, and glutamate. (Remember GABA is a major inhibitory NT)
Alcohol and Anxiety
The amygdala is involved in perception of stressful conditions and fear/stress response. Alcohol increases GABA activity and decreases output of downstream neurons leading to a depressed fear response and decreased anxiety.
Molecular pathway of stress relief
NPY is a anti-stress peptide and CRF is a pro-stress peptide. When CRF binds to its receptor it causes a downstream cascade in the cell which increases Adenylate Cyclase, activates PKA and CREB, and increase NPY gene transcription to decrease anxiety, so CRF production cedes.
What does chronic ethanol consumption do to the NPY/CRF negative feedback loop?
It disrupts the negative feedback loop and decreases NPY production, which will ultimately increase anxiety.
Pharmacokinetic tolerance to alcohol
There is a similar latency, but metabolism of alcohol is faster in a chronic user, so there is less alcohol in the blood at a given time. There is a higher level of ADH, CYP450, and ALDH in a chronic user.
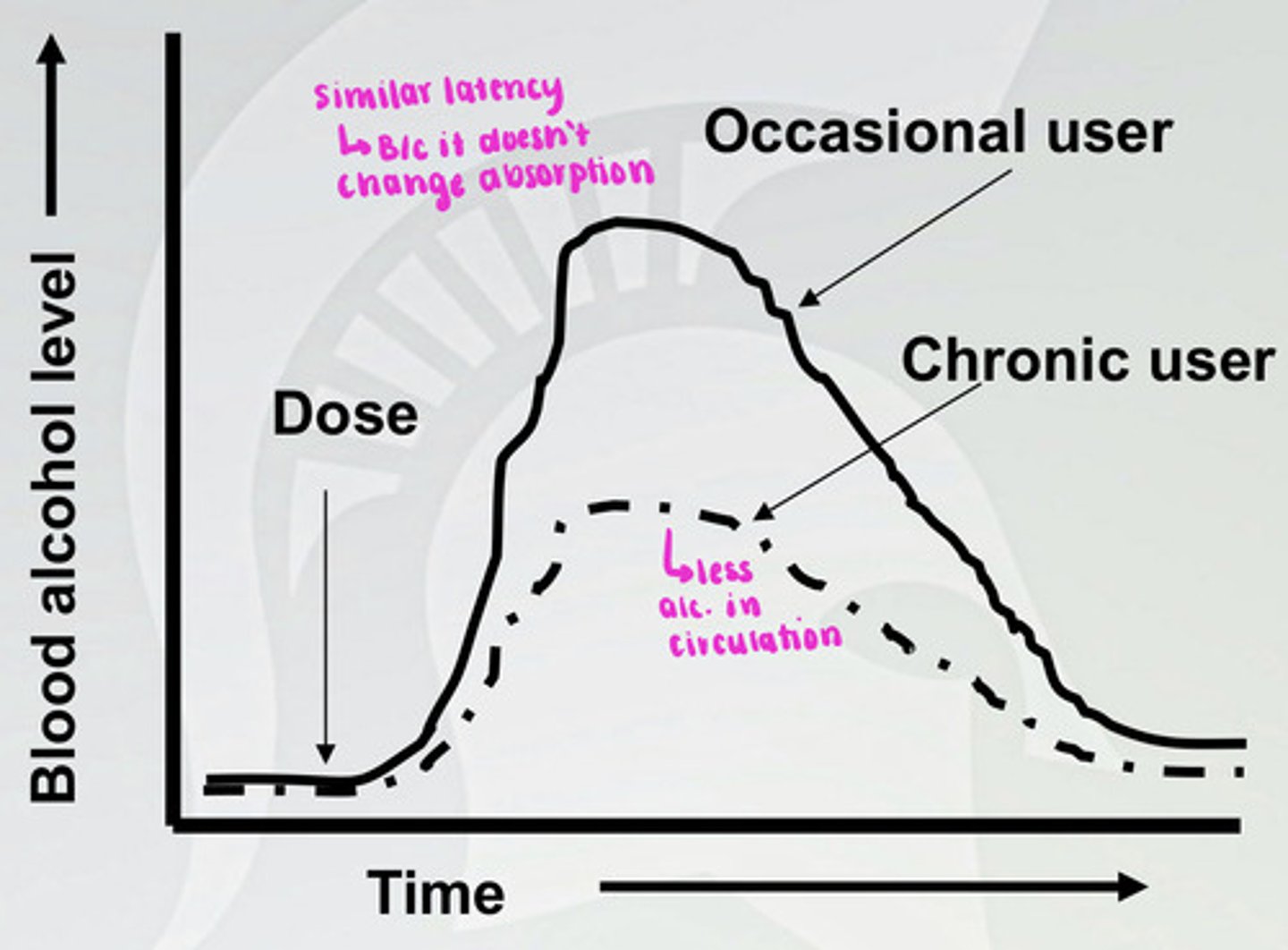
What is acute alcohol tolerance?
This happens within one time of using alcohol. The plasma concentration of alcohol goes up and comes back down so that you have the same concentration in your blood twice. However, anxiety and motor skills are higher on the downturn, because there is more signaling at receptor at decent.
What is the normal functioning at the synapse without alcohol in the system?
The GABAa receptor is not agonized and there is a normal level of Cl- and Ca2+ in the presynaptic neuron, this allows Glutamate to leave the cell and attach to the NMDA and AMPA receptors and cause excitation in the post-synaptic cell.
What happens to the neuron when alcohol is present in the system?
It agonizes the GABAa receptor, which increases Cl- and prevents a decrease in Ca2+. Thus, glutamate vesicles do not dock to the presynaptic membrane and glutamate does not bind to the NMDA and AMPA receptors on the post-synaptic neuron. Therefore, no excitation occurs in the post-synaptic neuron.
What happens to the neuron when alcohol is chronically present in the system?
Glutamate and Ca2+ voltage-gated channels are unregulated in the presynaptic neuron because it does not understand why the post-synaptic neuron is not getting excited. The post-synaptic neuron up-regulates NMDA receptors.
What happens at the synapse during alcohol withdrawal?
The presynaptic cell is no longer being hyper polarized, so the build-up of glutamate is finally released into the synapse. It binds to the NDMA receptors which have been unregulated, so there is A LOT of excitation in the post-synaptic cell. This is what causes the hangover symptoms and increased anxiety. The CNS goes from being depressed to OVER-stimulated.
What are the symptoms of alcohol withdrawal syndrome?
- Alcohol craving
- irritability, tremor, and nausea
- sleep disturbances
- high BP
- Rapid heart beat
- delirium tremors (DTs) in extreme cases
- agitation, confusion, hallucinations, nausea, and diarrhea
what are benzodiazepines?
- Helps with anxiety
- Narrow theraputic Index (a small concentration between therapeutic and toxicity)
- develop tolerance very quickly
- easy to get addicted
- BDZ is the shortened version
What are endophenotypes and how do they relate to alcoholism?
Behavior and genes put together that set people up for alcoholism which is passed down from generations. So, if you drink a lot with your friends and also have a genetic predisposition to alcoholism you are more likely to get addicted.
How do polymorphisms relate to alcoholism?
Polymorphisms are variations of the same gene. Certain polymorphisms of the genes that make ADH, ALDH, D2 and D4 (dopamine receptors) can make you more susceptible to alcoholism.
What are the four subunits of GABAa Receptor?
1. Alpha 2
2. Alpha 4
3. Beta 1
4. Gamma 1
(the GABAa receptors are pentamers)
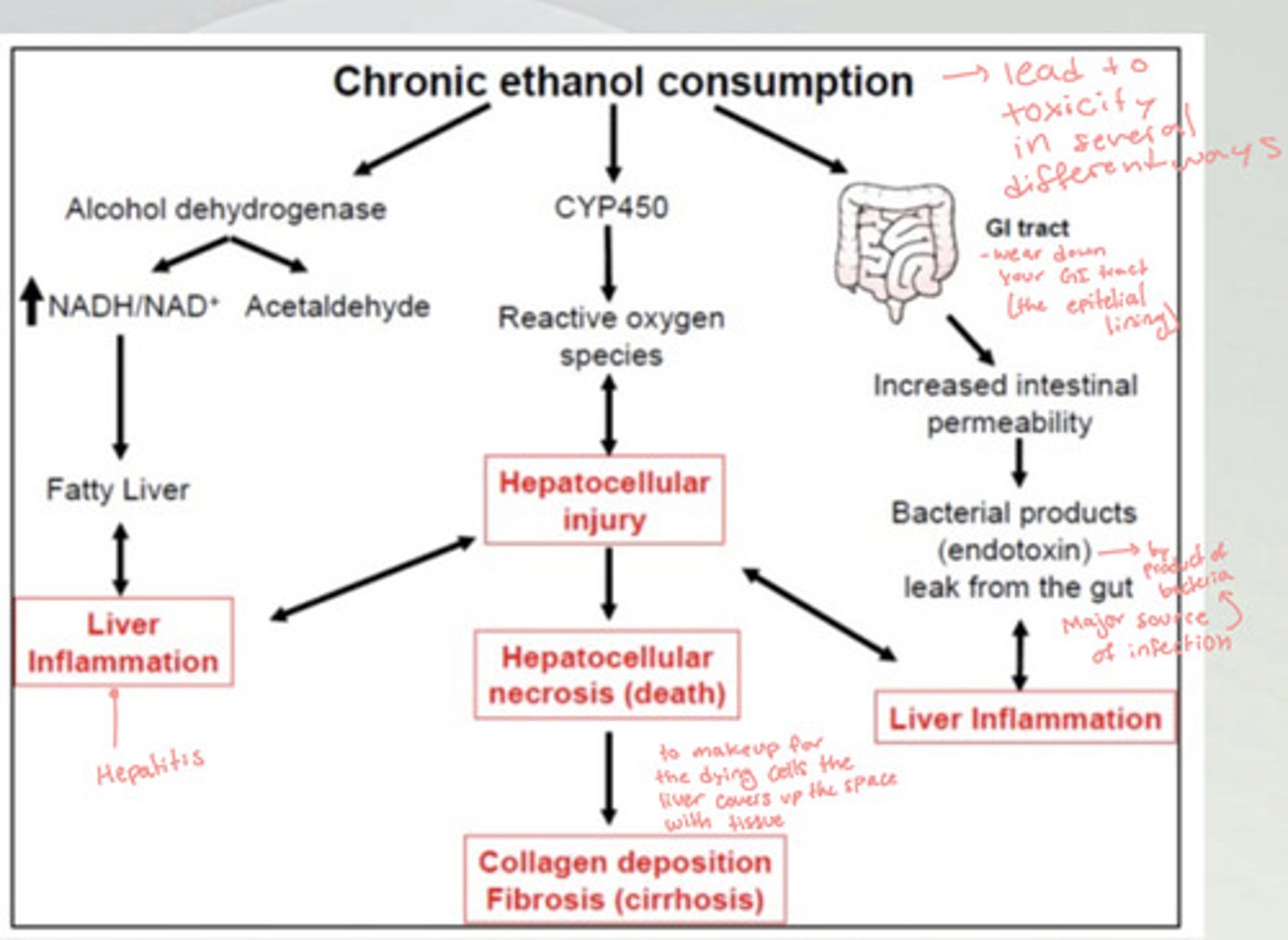
How does chronic alcohol consumption lead to liver inflammation (hepatitis)?
There is an upregulation in alcohol dehydrogenase, which increases NADH/NAD+ and acetaldehyde in the body. This causes a fatty liver and leads to liver inflammation. This can lead to a hepatocellular injury which leads to cirrhosis.
Another way this can happen is the chronic ethanol consumption wears down the epithelial lining of the GI tract, which increases intestinal permeability. This lets bacterial byproducts leak out from the gut and cause liver inflammation and infection which leads to hepatocellular injury and eventually cirrhosis.
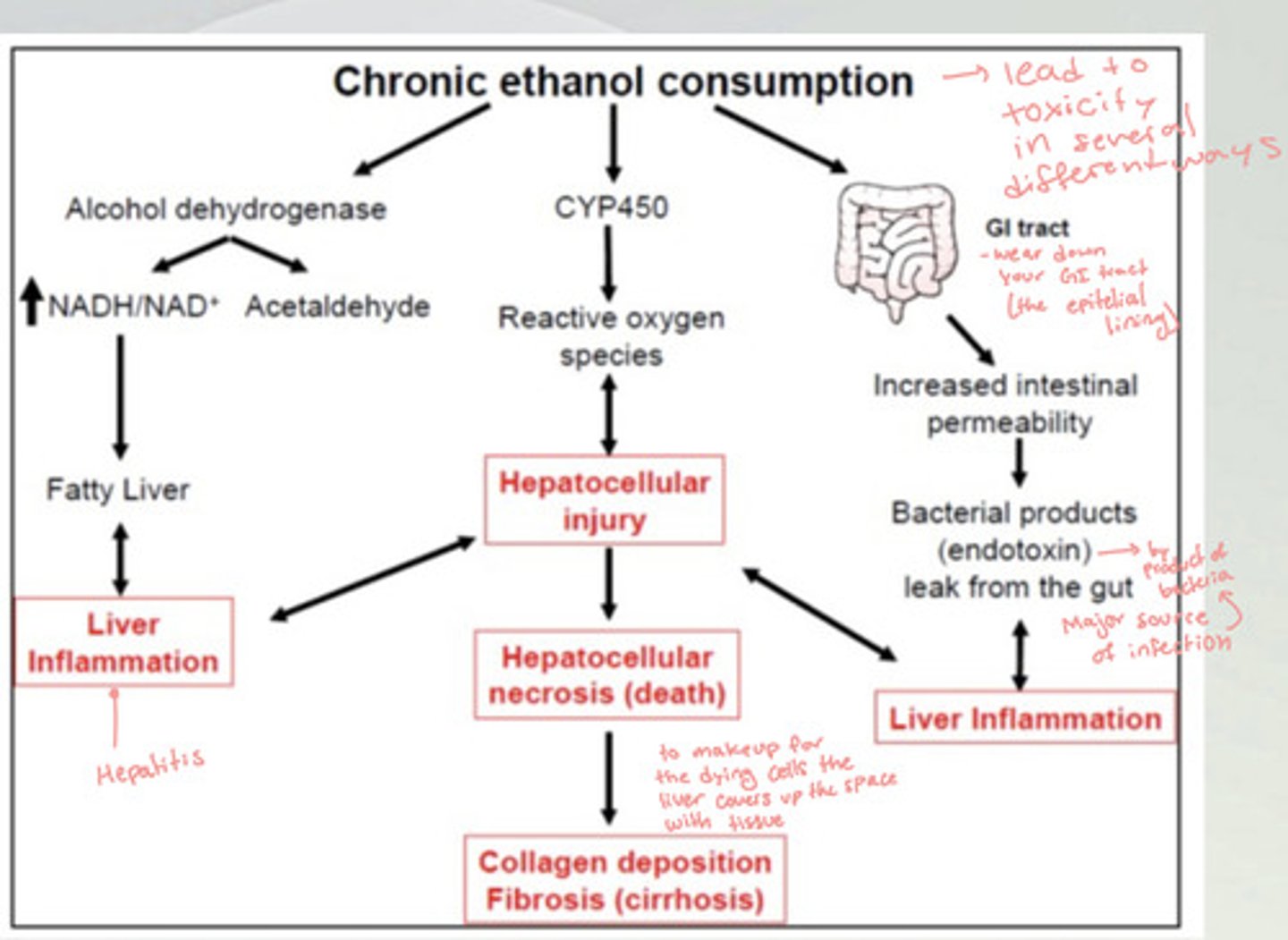
How does chronic alcohol consumption lead to Cirrhosis?
There is an increase in CYP450, which increases the levels of reactive oxygen species and can cause hepatocellular injury. The more injury there is the more reactive oxygen species. This leads to hepatocellular necrosis (death), which causes the collagen deposition fibrosis (cirrhosis), because the liver is making up for the dying cells by covering it up with collagen fibers.
Wrenicke's encephalopathy
A vitamin B1 (thiamine) deficiency that occurs with cirrhosis. This causes confusion, ataxia, and visual disturbances that are reversible with Thiamine administration.
Korsakoff's Psychosis
When you lose brain tissue caused by long periods of large amounts of alcohol intake. This is the irreversible loss of neurons.
Where do the endogenous ligands of GABA NTs bind on the GABAa receptors?
between the alpha and beta receptors
what subunits do Benzodiazepines bind on the GABAa receptors?
between gamma and alpha subunits
What does pentobarbital do to neurons?
It increases the GABA response. There is a larger influx of Cl- into the neurons causes a greater hyperpolarization.
Cross tolerance between alcohol and barbiturates?
The more barbiturates one consumes while drinking alcohol the more tolerant they become to the anti-anxiety effects of both drugs.
What are some benzodiazepines that people use for sleep disorders?
- Zolpidem (Ambien)
- Eszopiclone (Lunesta)
What is Gamma-hydroxybutyrate (GHB)?
- it is a byproduct of GABA metabolism and it increases the effects of alcohol
- it is similar anxiolytic to alcohol and benzodiazepines
- it has a short half-life (30 min)
- Synergistic effects with alcohol and produces amnesia at high doses
Propofol
- drug of choice for induction of anesthesia and for short-term surgical anesthesia. It is a powerful sedative and amnesia-inducing drug.
- Very safe and short-acting and there is a reduced "hangover" or drowsiness after use.
What were the tests for innate anxiety in mice?
- They were placed in an elevated plus maze, without anxiolytic drugs they would not go to the open-arms
- Novelty-suppressed feeding was when they were placed in a bin with food put out in the open in the middle of the bin they were less likely to eat the food if they were not given diazepam.
What are alpha 2 receptors involved with?
Anxiety
What do CB1 receptors target?
- motor activity
- thinking
- motor-coordination
- appetite
- short-term memory
- pain perception
- immune cells
What do CB2 receptors target?
- Gut
- Kidneys
- Pancreas
- Adipose Tissue
- Skeletal muscle
- bone
- eye
- tumors
- reproductive system
- immune system
- respiratory tract
- skin
- CNS
- Cardiovascular system
- Liver
What kind receptor is cannabinoid receptor?
A g-coupled protein receptor
what are signaling pathways linked to cannabinoid receptors?
Cannabinoids will bind to CB1 and CB2 receptors, which inhibits Ca2+ channels and activates K+ channels causing an influx of K+. This causes a decrease in AC and then a decrease in cAMP, which results in decreased gene transcription.
What are the two types of endocannabinoids and what do they do?
1. AEA (anandamide)- agonist at CB1 and CB2 receptors (Higher affinity for CB1)
2. 2-AG: agonist at CB1 anf CB2 receptors
Synthetic Cannabinoids
They have a higher potency and higher efficacy, but they are dirty and they hit a lot of receptors. A lot of these are still unregulated.
What does THC have a higher affinity for?
THC has a higher potency at CB1 receptors
What does THC do in the reward pathway?
THC binds to CB1 receptors in the VTA and block GABA release, this allows the dopamine neurons to release dopamine on D1 receptors in the prefrontal cortex and the nucleus accumbens. In turn the neurons in the prefrontal cortex release glutamate onto the Dopamine neurons in the VTA to keep the cycle going.
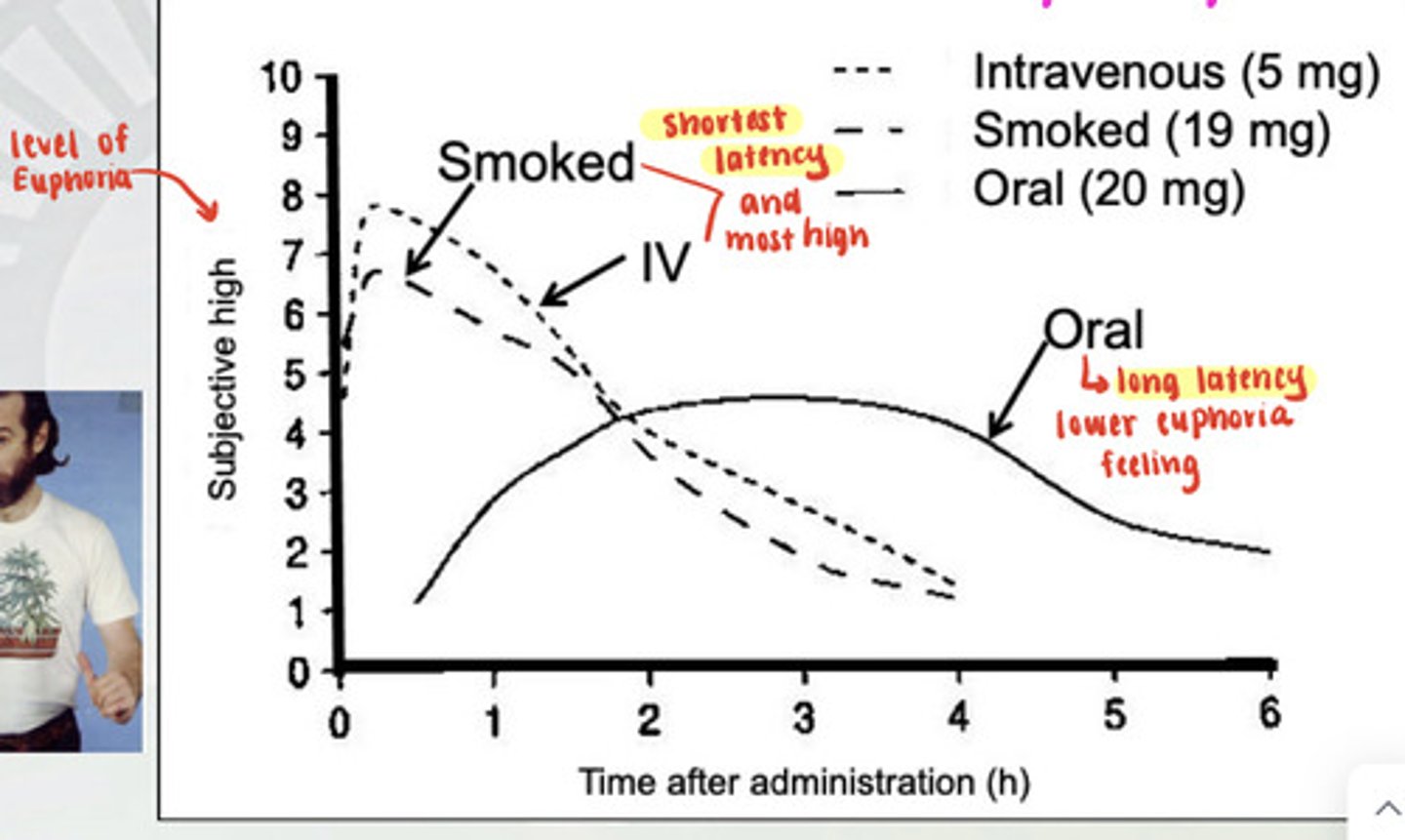
What are the routes of administration for cannabis and what are the latencies?
You can smoke it, take it orally, and take it intravenously. Smoking and IV have the shortest latency and make you the most high. Taking it orally has a lower euphoria feeling and a long latency.
How does the metabolism of THC work?
1. First pass: delta-9 THC is turned into 11-OH-THC by CYP 2C9
and then turned into THC-COOH by CYP 2C9
2. Second pass: THC-COOH has a glucose attached to it by glucuronyl transferase
* THC-COOH is in circulation for the longest time this is what shows up on drug tests.
What are the brain regions associated the physiologic responses to delta-9 THC activity?
- VTA-NAcc (reward pathway)
- NTS (nausea relief)
- Hypothalamus (appetite stimulation)
- Periaqueductal gray (pain pathway)
How do endocannabinoids do retrograde neurotransmission?
Glutamate is released from the presynaptic neuron, it binds to metabotropic and inotropic receptors on the postsynaptic cell which increases Ca2+ and releases anandamide into the synapse. The anandamide molecules attach themselves to the CB1 receptors on the presynaptic and decrease Ca2+ and increase K+. (this is not a cycle because of this reason)
How does THC suppress chemotherapy-induced nausea and vomiting?
The vagus nerve transmits a signal to the NTS which stimulates the CTZ area which causes vomiting and nausea. When someone takes delta-9 THC the THC binds to CB1 receptors in the presynaptic vagal nerve terminal in the NTS and inhibit the release of Glutamate into the synapse. Thus, it does not cause excitation in the postsynaptic neuron in the NTS and the NTS cannot stimulate the CTZ to cause the nausea and vomiting.
Hypothalamic Regulation of Feeding Behavior
THC binds to CB1 receptors in the Arcuate Nucleus which inhibits it from inhibiting the lateral hypothalamic area. The lateral hypothalamic area causes an increase in Orexin which is released into the VTA and this increases feeding behavior.
What does Orexin do?
it is a chemical that tells you when it is time to eat. THC causes the release of this even when you are not hungry, and that is why people get the munchies when they smoke weed.
CB receptors in pain and inflammation pathways (IMPORTANT)
- THC activates descending inhibition, which turns on the caudal raphe (5-HT mediated) inhibition of 2nd order. THC inhibits synaptic transmission to 2nd order neurons.
- THC acts on CB2 receptors to inhibit macrophages and microglia to suppress the inflammatory response to pain.
- THC blocks the neural transmission to second order neurons, which inhibits the pain pathway.
How does THC induce apoptosis?
THC binds to CB2 receptors in immune cells which leads to the activation of cascade, which induces apoptosis.
What is Crohn's Disease?
- Chronic inflammation of the ileum (last section of the small intestine) and the first part of the colon.
- caused by an inappropriate immune response to normal bacteria living in the gut.
- Can be hereditary
- Chronic diarrhea, intestinal bleeding, abdominal pain, weight loss, fatigue.
- Cannabis has been shown to improve Crohn's Disease Activity Index (CDAI)
How has cannabidiol impacted childhood epilepsy?
It causes a huge decrease in seizures
What is Sativex?
it is an oral mucosal spray that is used to treat spasticity multiple sclerosis. It is efficacious for preventing muscle spasms and pain.
What are the neural impacts of THC use?
- it can impair spatial learning and memory in mice. When mice were put into the Morris-Water Maze after taking THC they took more time to find the platform then when they were sober.
- It suppresses EEG Gamma oscillations; the older you are the better your brain can handle cannabis; you cannot remember as much when you are younger.
What is the schedule for marijuana?
It is a schedule 1 drug.
What does Dronabinol (Marinol) and Nabilone (Cesamet) do? (cannabis pharmaceutical)
- it is synthetic THC that is in sesame oil
- It is used for the cessation of nausea associated with cancer chemotherapeutics and appetite stimulation
- oral administration
- Dronabinol is marketed in the US and Nabilone is marketed in Canada.
What does Rimonabant do? (cannabis pharmaceutical)
- CB1 receptor agonist
- It is adjuvant to diet and exercise for treatment for obesity
- oral administration
- The FDA advisors rejected approval because of suicidal tendencies; causes powerful nausea
- This was discontinued
Naturally occurring opioid compounds
- Morphine
- Codeine
- Thebaine
- Papaverine
What is a opioid semi-synthetic compounds?
- Diamorphine (heroin)
What is a opioid synthetic compound?
Fentanyl and Methadone
What are the three classes of endogenous opioid peptides?
- Enkephalin
- beta-Endorphin
- Dynorphin
What do enkephalins and dynorphins do in the brain?
1. They are widely distributed in the brain (NAcc, Amygdala, cortex, and hypothalamus)
2. Found in high concentrations in the PAG and nerve fibers project to the raphe nucleus and descending pain inhibitory pathways
3. Enkephalins activate mu and delta opioid receptors
4. Dynorphins activate Kappa receptors
What do beta-endorphins do in the brain?
1. Largely only in the pituitary gland
2. Acts as a circulating hormone rather than a neurotransmitter. Typically released in response to stress. It comes from the same precursor as ACTH that stimulates the adrenal gland to release cortisol.
3. This peptide works on all three opioid receptors mu, delta, and kappa.
What opioid receptors are expressed on the soma of a neuron?
mu and delta (mostly delta)
What opioid receptors are expressed on the axon terminal of the neuron?
mu, delta, and gamma (mostly delta and gamma)
* Activation of all three receptors influences Ca2+ channel functioning
What does binding of morphine do mu and delta receptors?
1. morphine binds to a mu or delta opioid receptor (GPCR)
2. The beta and gamma subunits disassociate
3. Inhibits the Ca2+ channels from letting Ca2+ in
4. Activates K+ channels to let K+ out which causes a hyperpolarization of the cell.
* Positive ions are banned and also leave the cell
5. This causes inhibition of AC, then cAMP, then PKA, then CREB
6. leads to decreased gene expression
Where are the opioid receptors present in the brain?
opioid receptors are present in all regions of the brain, except K is not present in the prefrontal cortex and the Medulla
What are the effects of opiates?
- Euphoria
- sedation, sleepiness
- reduced body temperature
- reduced BP and heart rate
- pupil constriction
- constipation
- analgesia
What are the effects of opiate withdrawal?
- Cravings for more opiates
- irritability and restlessness
- sweating (increased body temp)
- increased blood pressure and heart rate
- pupil dilation
- diarrhea
- increased sensitivity to pain (hyperalgesia)
+ Withdrawal is a sign of physical dependence but not addiction
What are the pharmacokinetics of Opiates?
- Absorption: Variable routes of administration
- Distribution: Widely, including the brain
- Metabolism: large 1st pass (20-40% remaining, but you only need a little for any reaction) and glucuronidation. IV of morphine has a small latency M3G is not active but it lasts the longest in the blood.
- elimination
Metabolism of Heroine
1. Heroine to 6-MAM (1st hydrolytic step [1st pass])
2. 6-MAM to morphine (2nd hydrolytic step [1st pass])
3. Morphine to M3G (glucuronidation [2nd pass])
* IV has a small latency, huge spike; and rapid breakdown
* Intranasal: not much gets into the plasma concentration
* metabolism does not match the psychoactive reactions to the drug
![<p>1. Heroine to 6-MAM (1st hydrolytic step [1st pass])</p><p>2. 6-MAM to morphine (2nd hydrolytic step [1st pass])</p><p>3. Morphine to M3G (glucuronidation [2nd pass])</p><p>* IV has a small latency, huge spike; and rapid breakdown</p><p>* Intranasal: not much gets into the plasma concentration</p><p>* metabolism does not match the psychoactive reactions to the drug</p>](https://knowt-user-attachments.s3.amazonaws.com/3544a7d2-bac6-467b-85ba-f88584c89dba.image/jpeg)
What is Mu1 opioid receptor responsible for?
Analgesia
What is the Mu2 opioid receptor responsible for?
- sedation
- vomiting
- respiratory depression
- pruitus
- euphoria
- anorexia
- urinary retention
- physical dependence
What is the delta opioid receptor responsible for?
Analgesia and spinal analgesia
What is the Kappa opioid receptor responsible for?
- analgesia
- sedation
- dyspnea
- psychomimetic effects
- pupil constriction
- respiratory depression
- euphoria
- dysphoria
- dyspnea
What are Agonists of the opioid receptors?
- Morphine
- Codeine
- Fentanyl
- Meperidine
- Methadone
What receptors does Morphine target?
mu and K (weak agonist for K)
What receptors does fentanyl target?
mu
What receptors does methadone target?
mu
What are opioid receptor antagonists?
Naloxone, naltrexone
What opioid receptors do naloxone and naltrexone target?
mu, delta (weak antagonists), and Kappa
what are distinct characteristics of heroin compared to morphine?
heroin passes the blood-brain barrier faster than morphine, but then heroin is converted to 6-MAM and morphine to work at opiate receptors.
What do Codeine, Oxycodone, and Hydrocodone do?
They all work as analgesics and are converted to morphine after administration through dealkylation.
What does Fentanyl do?
- it has a high potency
- it does not get converted to morphine it acts by itself. It has a very different structure.
Methadone
- it is synthesized as an analgesic
- used to treat opioid use disorder
- drug craving reduced with time
- There is cross-tolerance with morphine and heroin
- It causes lower peaks and valleys, so it keeps people feeling more normal. It has lower highs, but also not as bad withdrawals.
- You have to routinely administer it.
Naloxone and Naltrexone
- opioid antagonists
- Administered as a recovery agent not as a deterrent
- Nalaxone (Narcan) is an inhalant used to treat opioid overdose
- Naltrexone- Opioid and alcohol addiction . It blocks all there receptors mu > delta > Kappa. Given as a subcutaneous injection that lasts for a month.
Suboxone treatment for opioid use disorder
- partial agonist at Mu
- It has a much larger effect when taken intravenously, so it could provide more euphoria than taking it orally. (addicts are more likely to use it for this purpose)
- When taken orally it suppresses withdrawal and cravings, and when taken through IV it blocks naloxone from preventing euphoria due to it blocking receptors.
What brain areas does morphine activate?
VTA then the dopamine neurons which activate the nucleus accumbens neurons. Basically it activates the reward circuit.
How does morphine cause disinhibition of VTA neurons?
Morphine inhibits GABA interneurons from releasing GABA so the dopamine projection neurons are DIS-inhibited (they fire on the NAcc and trigger the reward pathway)