Electron configuration
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Ms
The spin quantum number, can have 2 values: +1/2 and -1/2. + is up and - is down.
Pauli Exclusion Principle
No two electrons can have the same four quantum numbers. Results in a specific orbital having ONLY 2 electrons one with a + spin and - spin.
Effective nuclear charge (Zeff)
The net charge experienced by an electron in a multi-electron atom, accounting for shielding by other electrons. It is calculated as the Zactual - Electron Shielding = Zeff
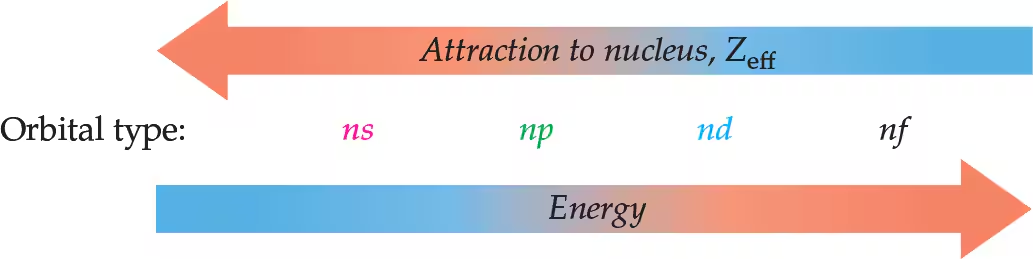
Zeff and Energy of orbitals graph
Electron configuration
A description of which orbitals in an atom are occupied by electrons
Aufbau Principle
The principle that electrons fill atomic orbitals starting from the lowest energy level to the highest. This is used to determine the electron configuration of an atom.
Ground-state electron configuration
The electron configuration of an atom in its lowest energy state, showing the distribution of electrons among the atomic orbitals.
Degenerate
orbitals that have the same energy level.
Hund’s rule
if two or more degenerate orbitals are available one electron goes in each until all are half full