Ionic Bonding
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Valence Electrons
Electrons in the outer most energy level of an atom.
Metals
These tend to lose electrons and are generally located on the left side of the periodic table.
Non-metals
These tend to gain electrons and are generally located on the right side of the periodic table.
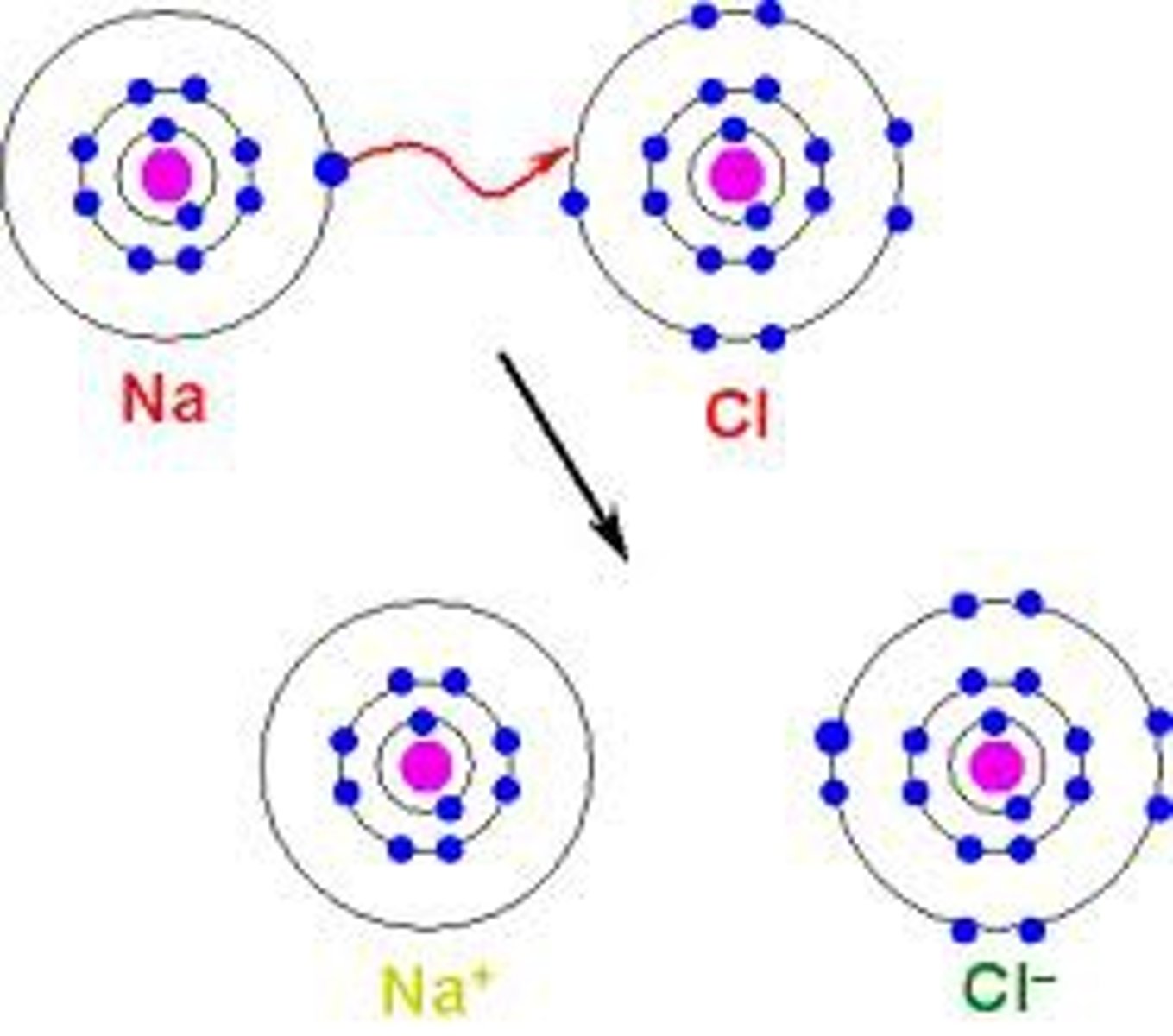
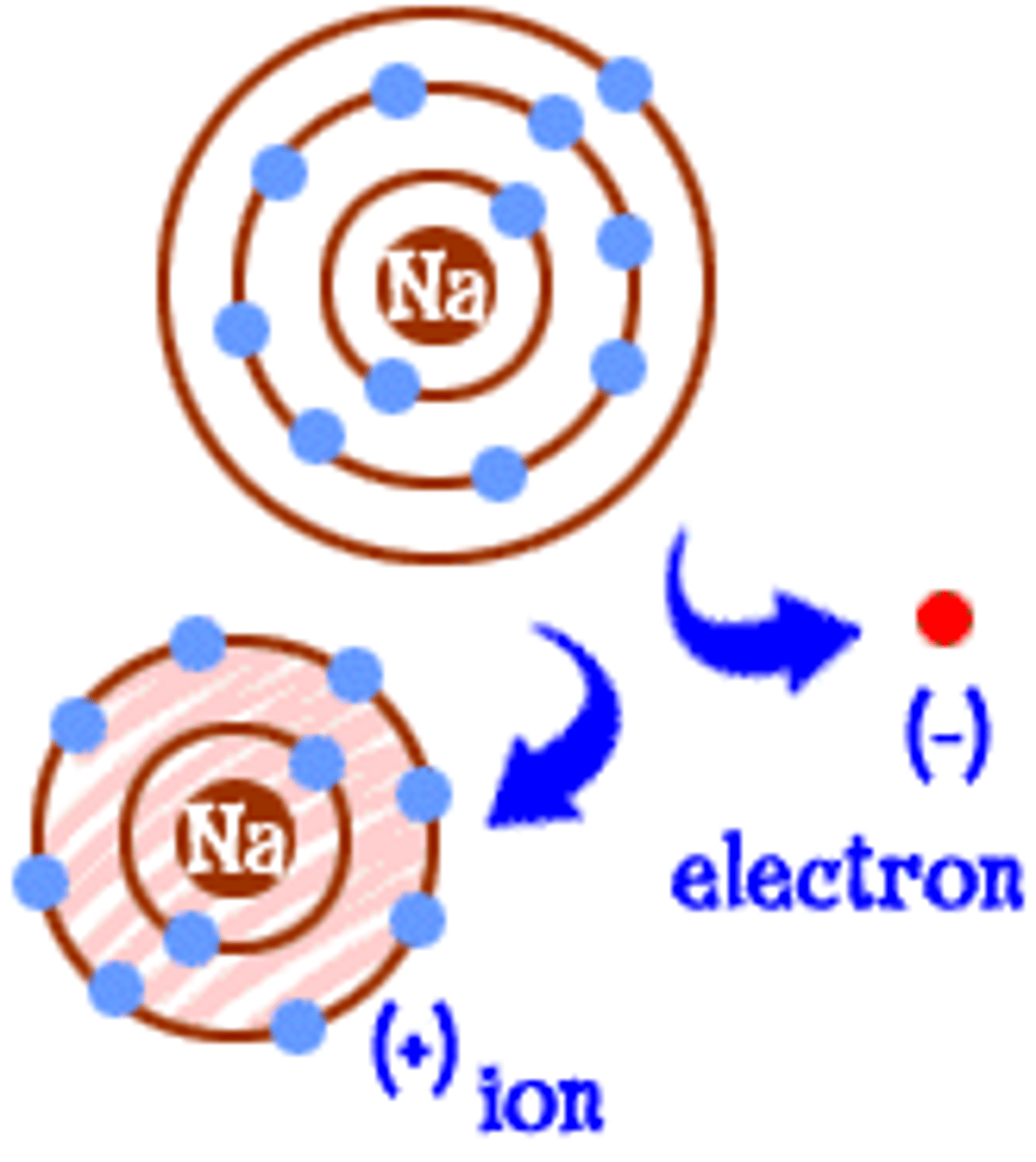
Ionic bond
This bond occurs between a metal and a nonmetal. It occurs when one or more electrons transfer from one atom to another.
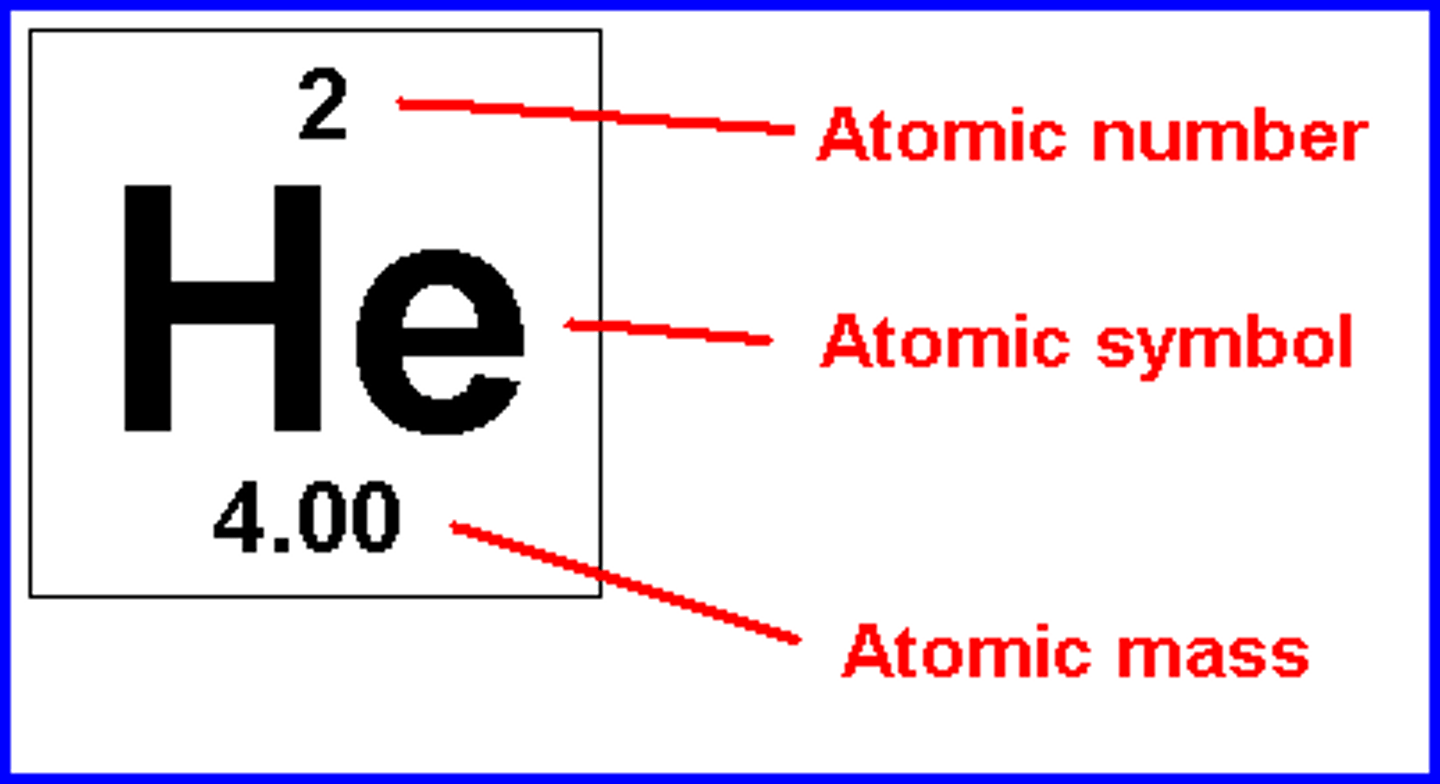
Symbol
The one or two letter abbreviation for an element.
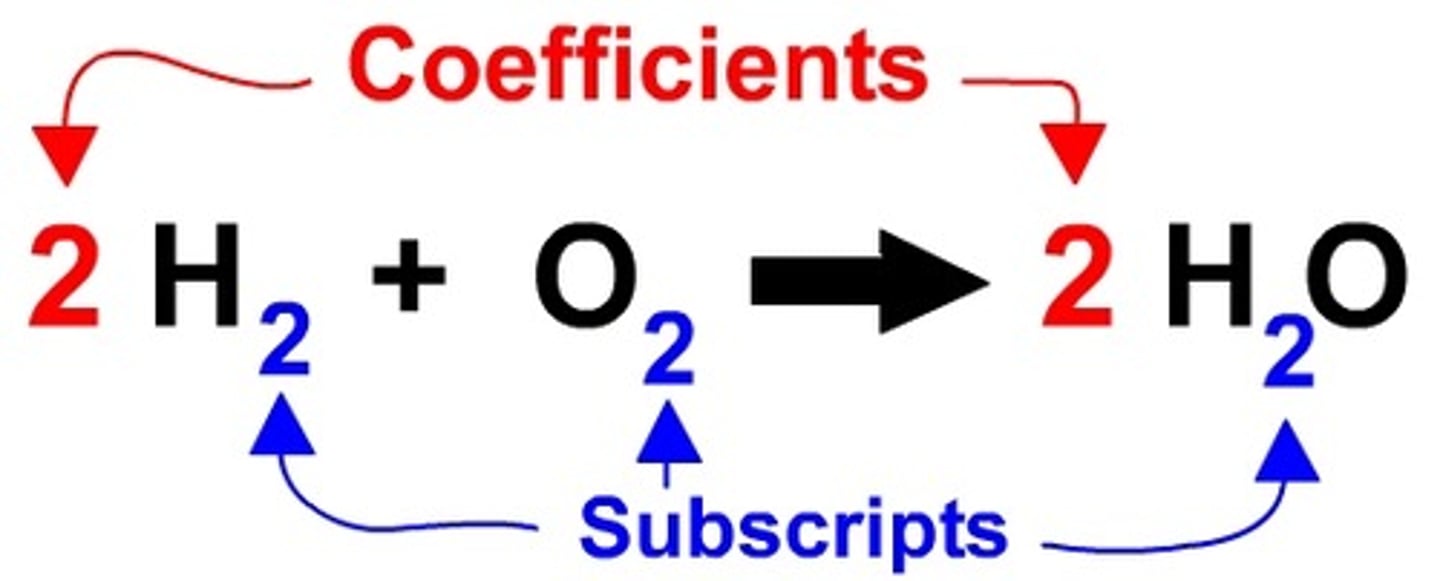
Chemical Formula
Contains symbols and subscripts and tells us what type of atom and how many atom the compound is made of.
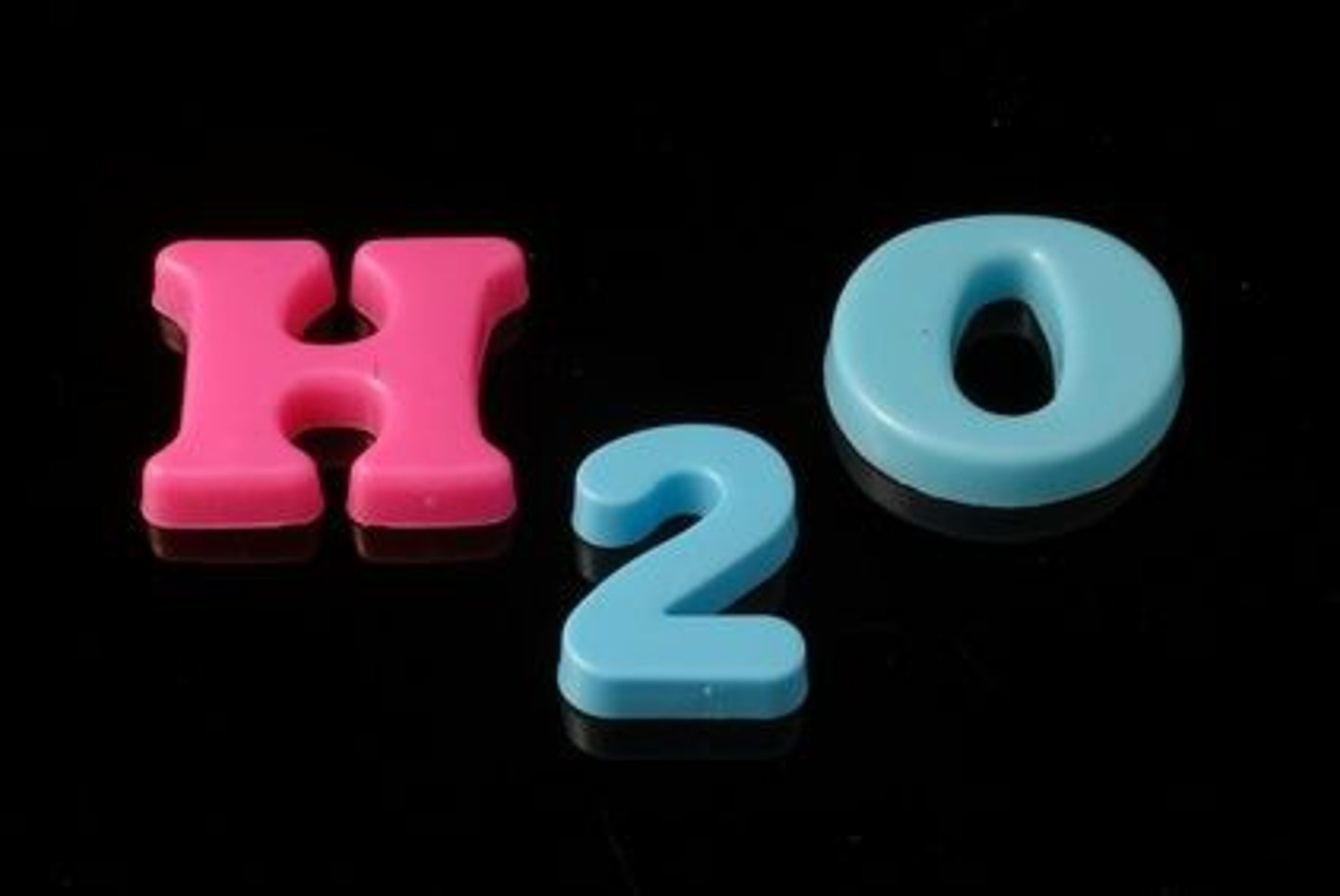
Compound
This forms when two or more elements bond to each other.
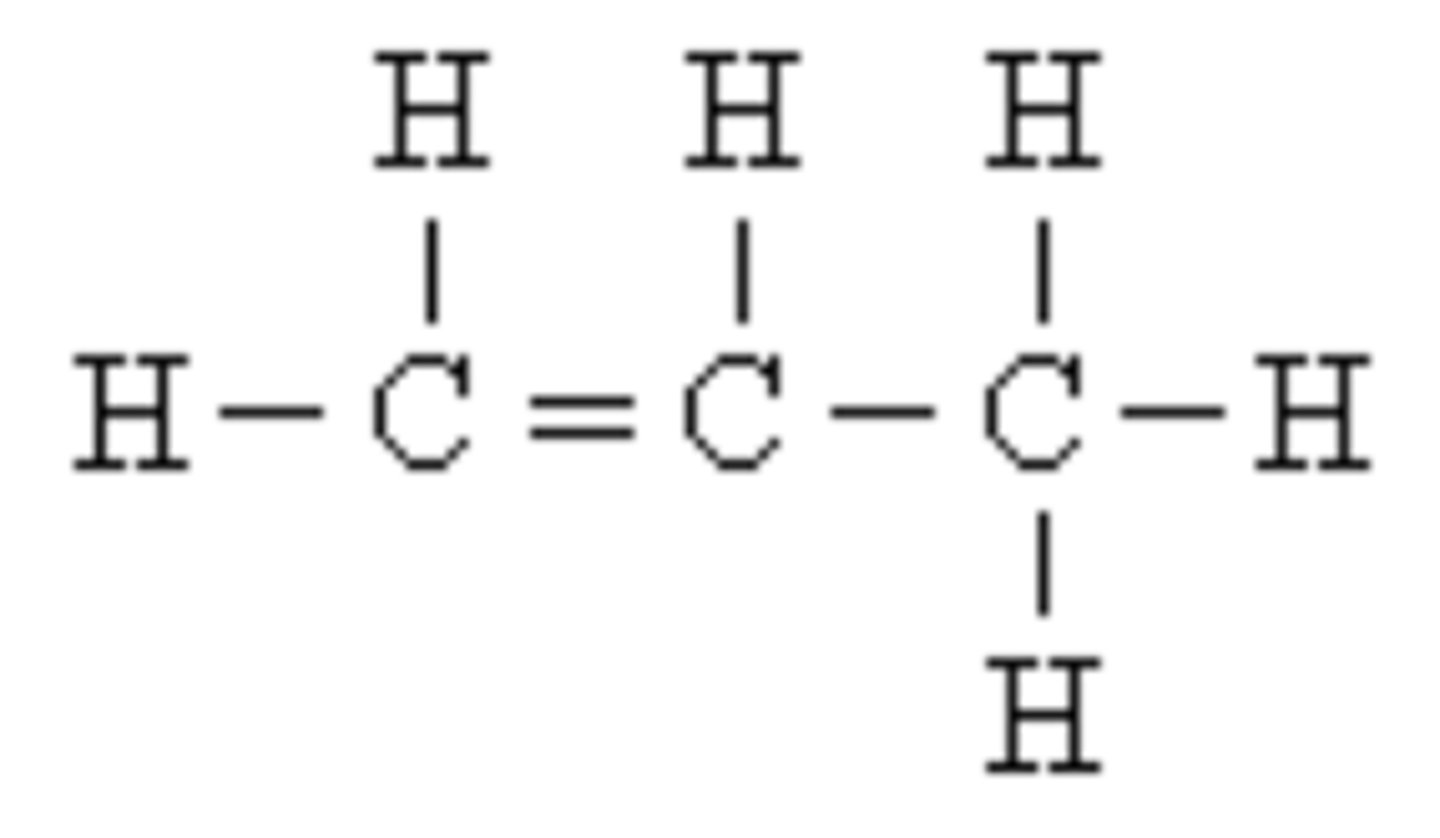
Ion
An atom or molecule with a charge because it has lost or gained electrons.
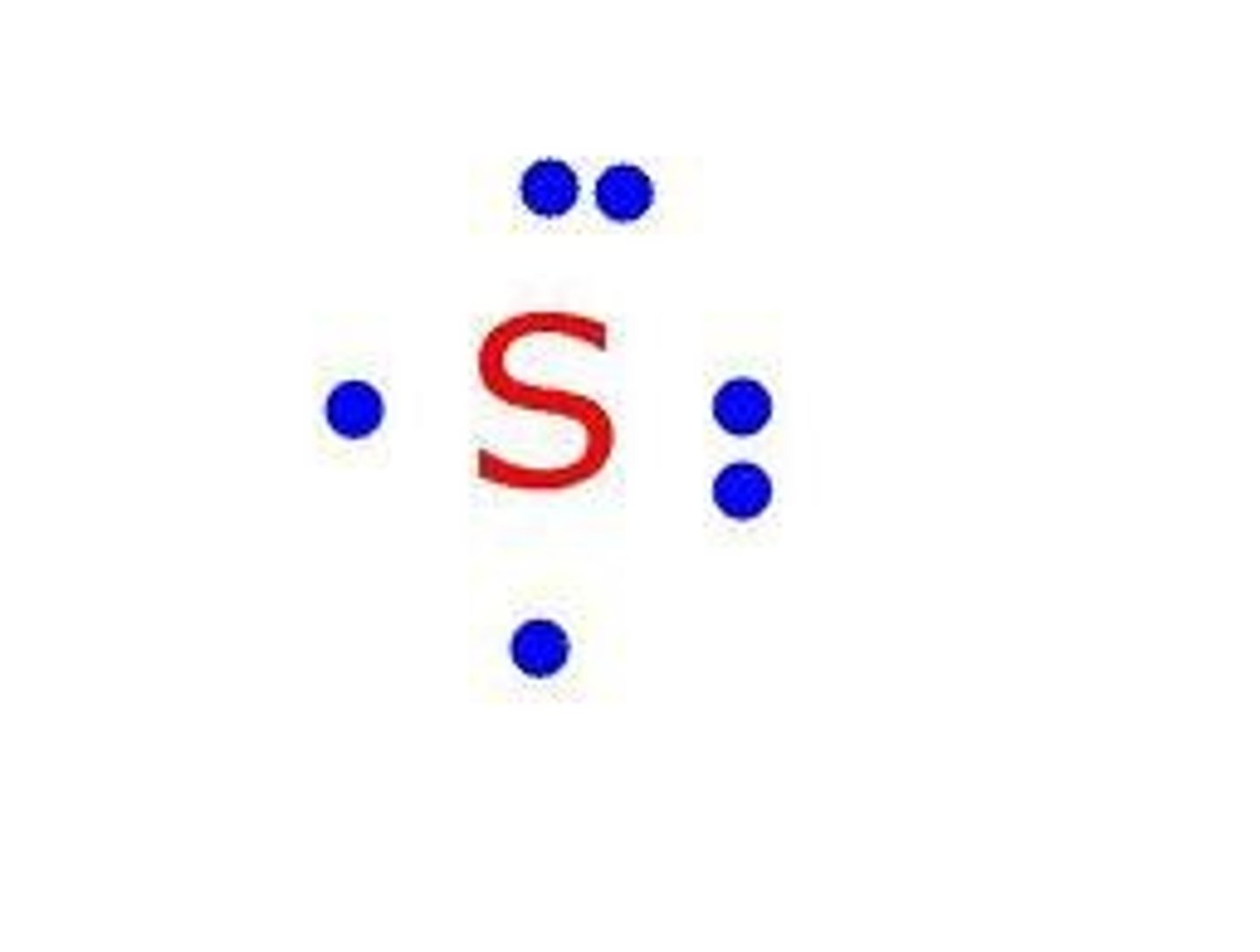
Electron dot diagram
This diagram is used as a represent the outermost electrons (valence electrons) in an element.
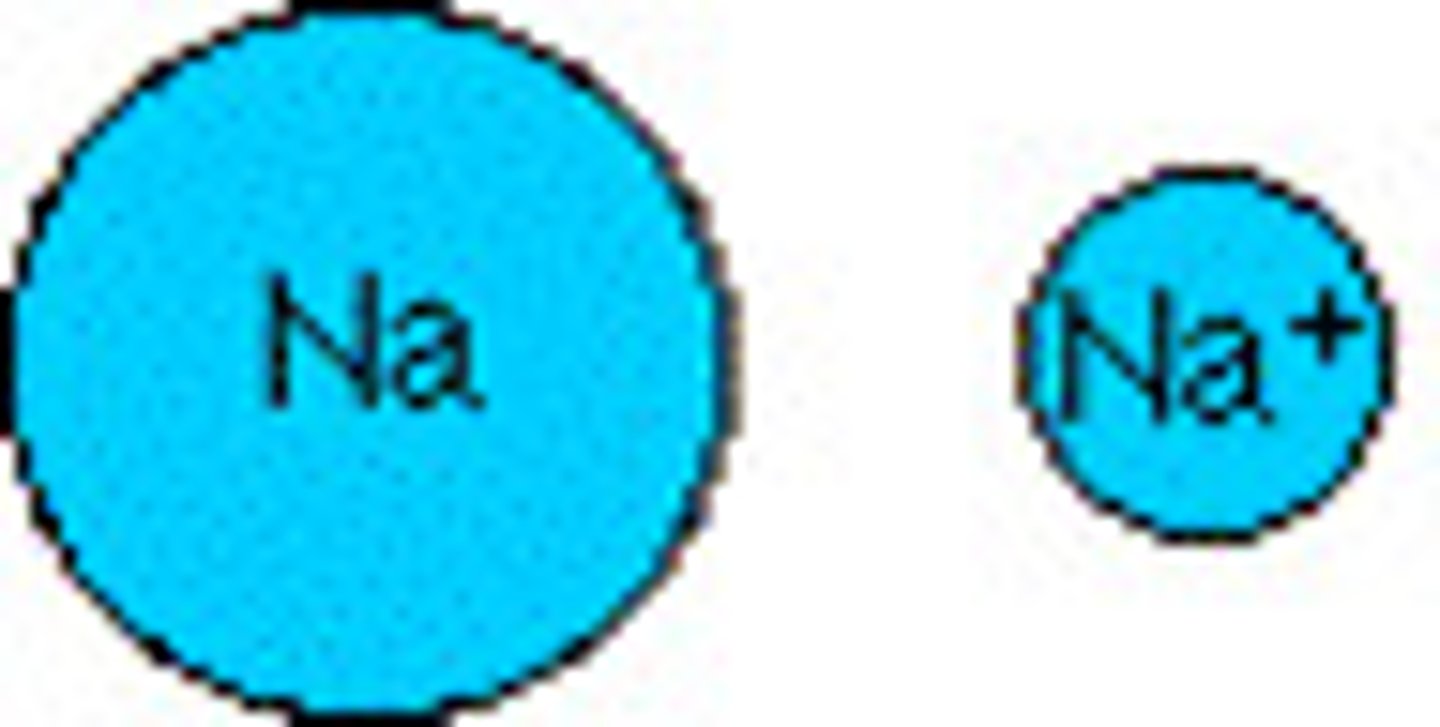
Cation
An ion with a positive charge
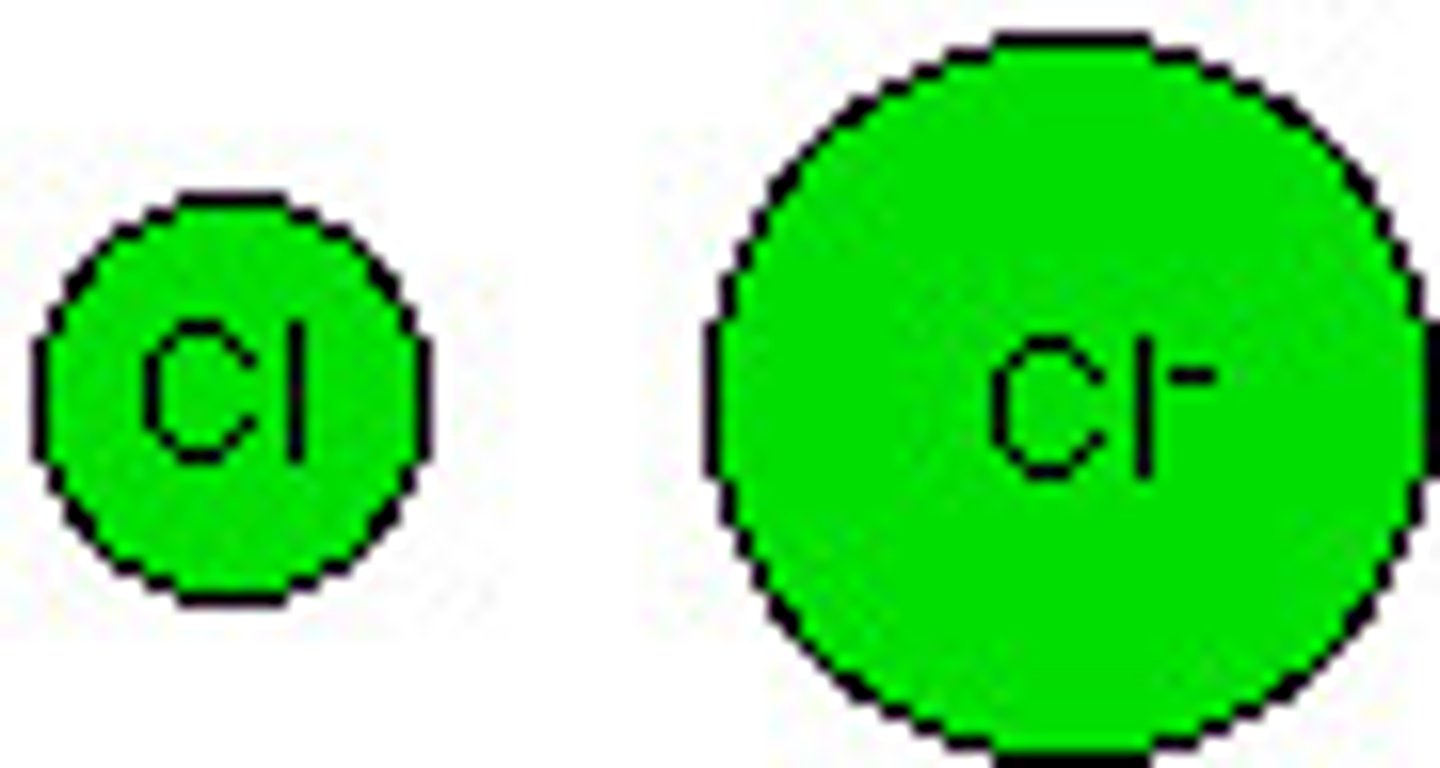
Anion
An ion with a negative charge
Octet
A full outer energy level with 8 electrons.

Subscript
Found in the lower right of a chemical formula. This tells us how many of each atom are in a compound.
+1
Group 1 elements will form an ion of this amount of charge.
+2
Group 2 elements will form an ion of this amount of charge.
+3
Group 13 elements will form an ion of this amount of charge.
-1
Group 17 elements will form an ion of this amount of charge.
-2
Group 16 elements will form an ion of this amount of charge.
-3
Group 17 elements will form an ion of this amount of charge.
-ide
The suffix added to an anion's name
Superscript
Found in the upper right of the ion symbol. This tells us the charge of the ion.