Proteins
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Monomer of a protein
amino acids- made of a central carbon atom, an amino group, a carboxyl group and a R group that varies
Dimers of a protein
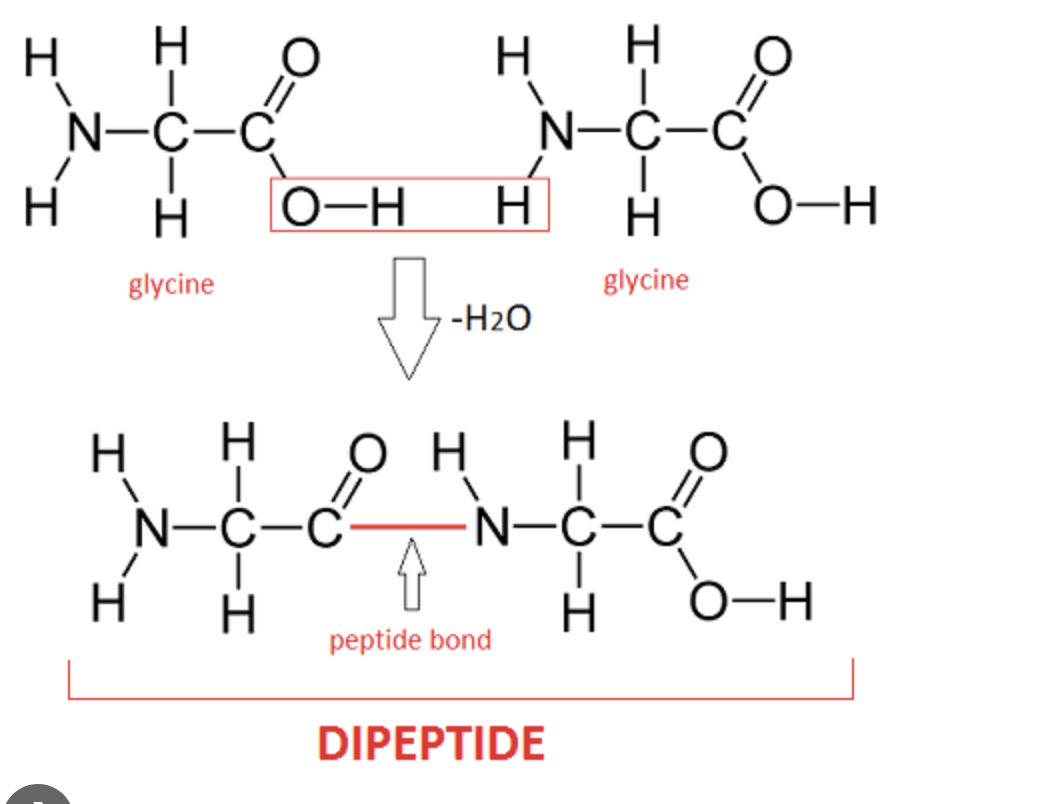
dipeptides- two amino acids linked together
Polymers of a protein
the polymers of proteins are peptides- made of repeating monomers (amino acids), bonded by peptide bonds
Animals rich in protein
eggs, chicken breasts, turkey breasts
Plants rich in protein
Chickpeas, peas, peanut butter
Six main functions of a protein
The 6 major functions of proteins in a cell are metabolism, support, transport, defense, regulation and motion.
Metabolism
enzyme proteins bring reactants together and speed up chemical reactions in cells. An example of metabolism is when your body breaks down and builds up proteins.
Support
Support is in some proteins. Examples of support are keratin for hair and nails and collagen for ligaments, tendons and skin.
Transport
An example of transport is hemoglobin which transports oxygen to tissue and cells.
Defense
In our immune systems antibodies are proteins that combine with antigens. The antibodies bind and prevent antigens from destroying cells and upsetting homeostasis.
Regulation
some hormones are proteins that regulate how cells behave and they can serve as messengers that influence cell metabolism. An example is insulin which regulates how much glucose is in the blood and cells.
Motion
proteins allow parts of cells to move and cause muscles to contract. Examples are actin and myosin.
Primary structure
all proteins have a primary structure, linear structure of amino acids in a polypeptide chain
Secondary structure
either a alpha helix or beta pleated sheet structure,
Bonds:
-hydrogen bonds (formed between amino group and carboxyl group)
Examples: silk, collagen, keratin
Tertiary/3rd level structure
globular shape (ball) caused by R groups interacting
Bonds:
-disulfide bonds (strongest, formed between sulfur atoms of two cysteine amino acids)
-Hydrogen bonds (occur between polar side chains of amino acids)
- ionic bonds (formed between oppositely charged amino acid side chains)
-hydrophobic interactions (non polar amino acid side chains clump together to minimize water contact)
Examples: enzymes: catalase, lactase + insulin, glycogen, myoglobin
Quaternary/4th level structure
2 or more globular structures linked together
Bonds
-most commonly hydrogen bonds because polypeptides are being linked together
Examples: hemoglobin, antibodies
Structural characteristics of a typical amino acid
all have a central carbon, hydrogen, amino group, carboxyl gorup and R group that differs in every amino acid
The connection between genes and proteins. How many proteins are found in humans ?
A gene is a section of DNA that contains the instructions for building a specific protein.
Between 20,000 and 100,000 proteins are found in humans.
What are essential amino acids? How many of the 20 different amino acids are considered “essential” to our diet?
Essential amino acids are essential because we don't make them. They’re about half of the 20 different amino acids. = 10
.Where in the cell are proteins synthesized?
The Ribosomes
Explain the “Central Dogma” of Biology
The transfer of genetic information from DNA to RNA (mRNA) to a protein
(ribosomes to be turned into proteins)
It's called “Central dogma of molecular Biology” because of its universality and how it happens to all living things.
Process by which information flows in living cells- DNA turns to RNA into proteins
DNA information is picked up by mRNA
RNA is the messenger that carries the instructions to the cells
Transcription
genetic information is being carried to the mRNA
Translation
The mRNA picks up the genetic code from the DNA and brings it to the ribosome to make proteins
What is an R group?
The R group represents the side chain that varies between different amino acids so no two amino acids have the same R group, influencing their properties and functions.
What is the role of an enzyme?
To speed up chemical reactions.
By seeding up they can build up or break down. ️
The role of an enzyme is to build up and break down molecules.
How do enzymes work
Substrate binds to the enzyme’s active site.
The enzyme causes a reaction (catalyzes) which converts the substrate to a product
Products are released, and the enzyme is ready for another reaction.
What variables affect the rate of an enzyme reaction?
increase in temperature
increase of decrease in pH
substrate concentration
enzyme concentration
the presence of inhibitors or activators
How do these factors affect the rate of an enzymes reaction
(temperature) increases but slows down if gets too far away from optimum
(pH) increases but slows down if gets too far away from optimum
(enzyme concentration) Increases at maximum but if not slows down
(substrate concentration) Increases at maximum but if not slows down
(presence of inhibitors) slows down the reaction
(presence of activators) increases the reaction
active site
The active site is a specially shaped area of the enzyme that fits around the substrate
conformation
The 3D shape of a molecule
ribosome
Where proteins are made → made by translating RNA into amino acid sequences.
amino group
A functional group (NH2) in amino acids, involved in peptide bond formation.
carboxyl group
A functional group (-COOH) in amino acids, can lose a hydrogen ion and forms peptide bonds.
denature
When a protein loses its shape and stops working
Substrate
the biological molecules that enzymes work on
alpha helix
A coiled structure in proteins formed by hydrogen bonding.
beta sheets
A protein structure where polypeptide chains fold into strands.
Peptide Bond
A covalent bond linking amino acids in a protein.
Dipeptide
A molecule consisting of two amino acids linked by a peptide bond.
Optimum temperature
the temperature at which the activity of an enzyme is at its highest
Optimum pH
the specific pH value at which the activity of an enzyme is at its highest
What type of bonding causes the formation of an alpha helix or a beta sheet
Hydrogen bonds
What type of bonding causes the formation of a protein’s Tertiary structure?
Disulfide bonds, hydrogen bonds, ionic bonds, and hydrophobic interactions
Identify the type of bond found in a protein’s primary structure?
The primary structure of a protein is held together by peptide bonds. These are covalent bonds formed between the amino group of one amino acid and the carboxyl group of another amino acid during protein synthesis.
Explain how a huge number of different proteins can be produced from just 20 different amino acids
Variability in Sequence: The order in which the 20 amino acids are arranged in the protein chain is what makes each protein unique.
Length of the Chain: Proteins can be made of hundreds or thousands of amino acids. This means there are an incredibly large number of possible combinations of 20 amino acids in long chains.
What is the name of the process that produces a dipeptide?
Dehydration synthesis
Co Factors
A cofactor is a non-protein molecule that is essential for an enzyme to function properly
Co Enzymes
A coenzyme is a biological molecule that enhances the enzymes function and attaches at the active site