Intermolecular and Intramolecular Forces in Chemistry: Properties of Solids and Liquids
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Intermolecular Forces
These forces exist between molecules and influence the physical properties of the substance.
Ion-ion Interactions
The force of attraction between two oppositely charged ions governed by Coulomb's law.
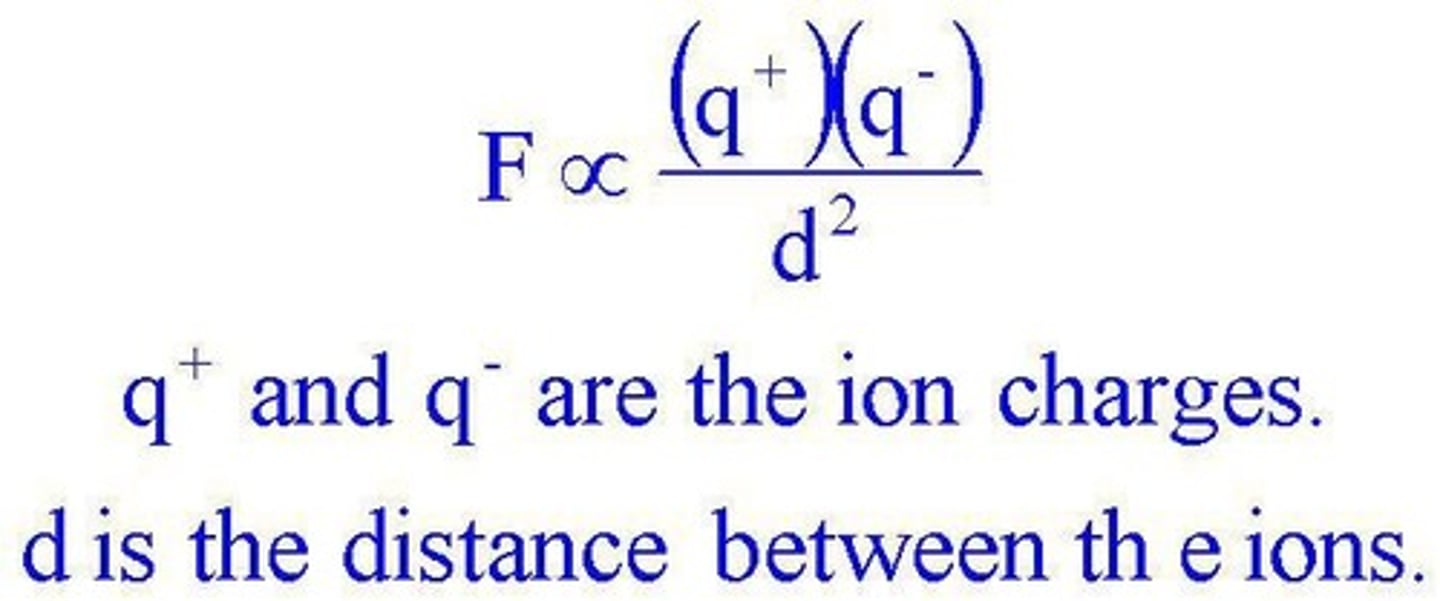
Coulomb's Law
Determines the melting and boiling points of ionic compounds; higher attraction forces lead to higher melting and boiling points.
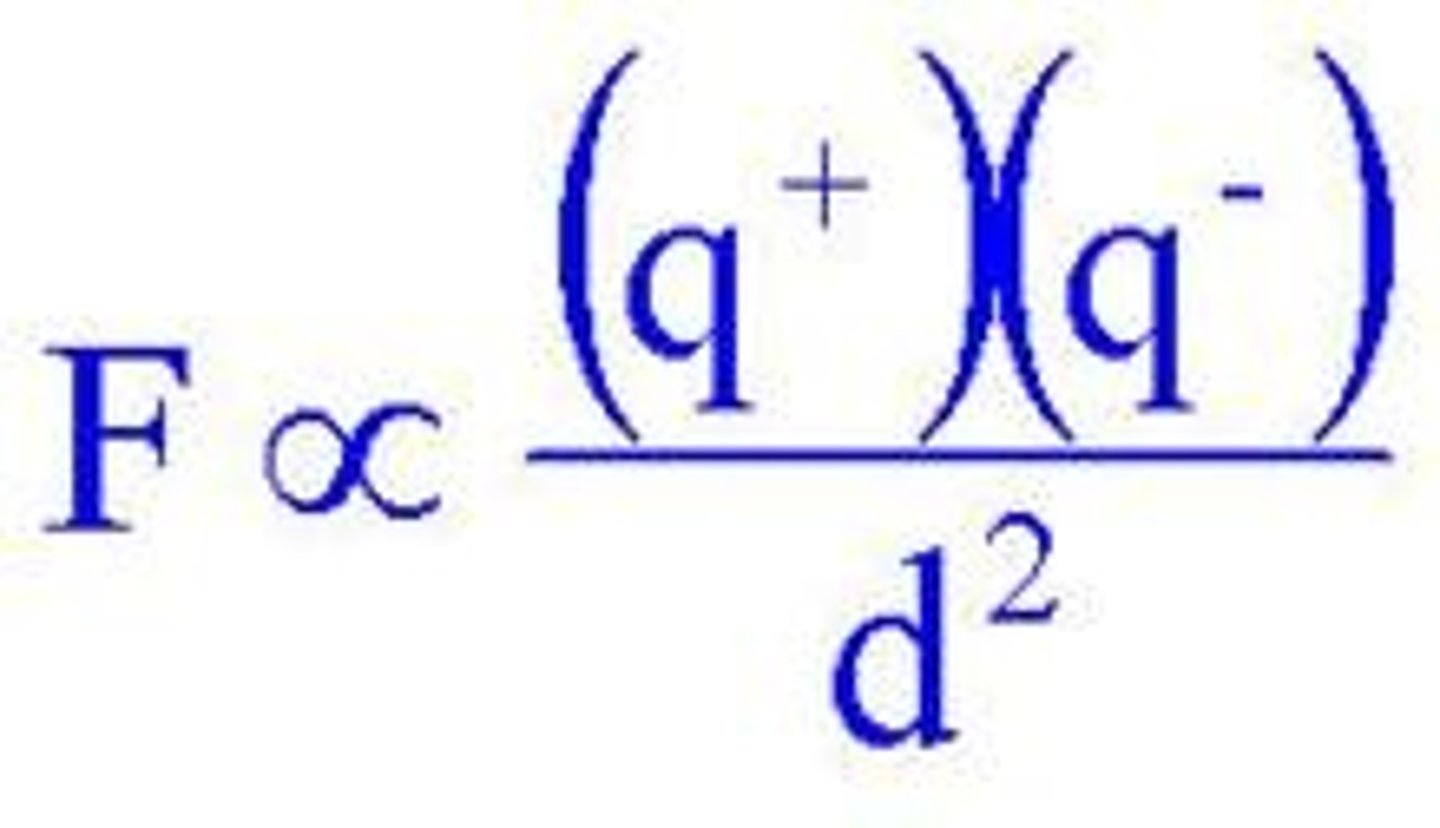
Ion-dipole Interactions
Attractive force between an ion and a polar molecule.
Dipole-dipole Interactions
Occurs only for molecular compounds that are polar.
Hydrogen Bonding
A special type of dipole-dipole interaction present in molecules having hydrogen atoms attached to N, O, F.
London Dispersion Forces
Also called van der Waals interaction; the weakest of the intermolecular forces present in all molecules.
Trend for IMFA Strength
Ion-ion (intramolecular) > ion-dipole > H-bonding > dipole-dipole > London dispersion force.
Molar Mass and Boiling Point
Generally, the stronger the intermolecular forces, the higher the boiling point.
Molecular Shape and Boiling Point
Branched versus linear structures affect boiling points.
Higher Charge and Melting Points
The higher the charge of the ion, the higher the melting/boiling points.
Smaller Ions and Melting Points
For ions with similar charge, smaller ions have higher melting/boiling points.
Polar Molecules
Molecules that have a dipole moment due to the presence of polar bonds.
Non-polar Molecules
Molecules that do not have a dipole moment and only exhibit London dispersion forces.
Weakest Intermolecular Force
London Dispersion Forces are the weakest of the intermolecular forces.
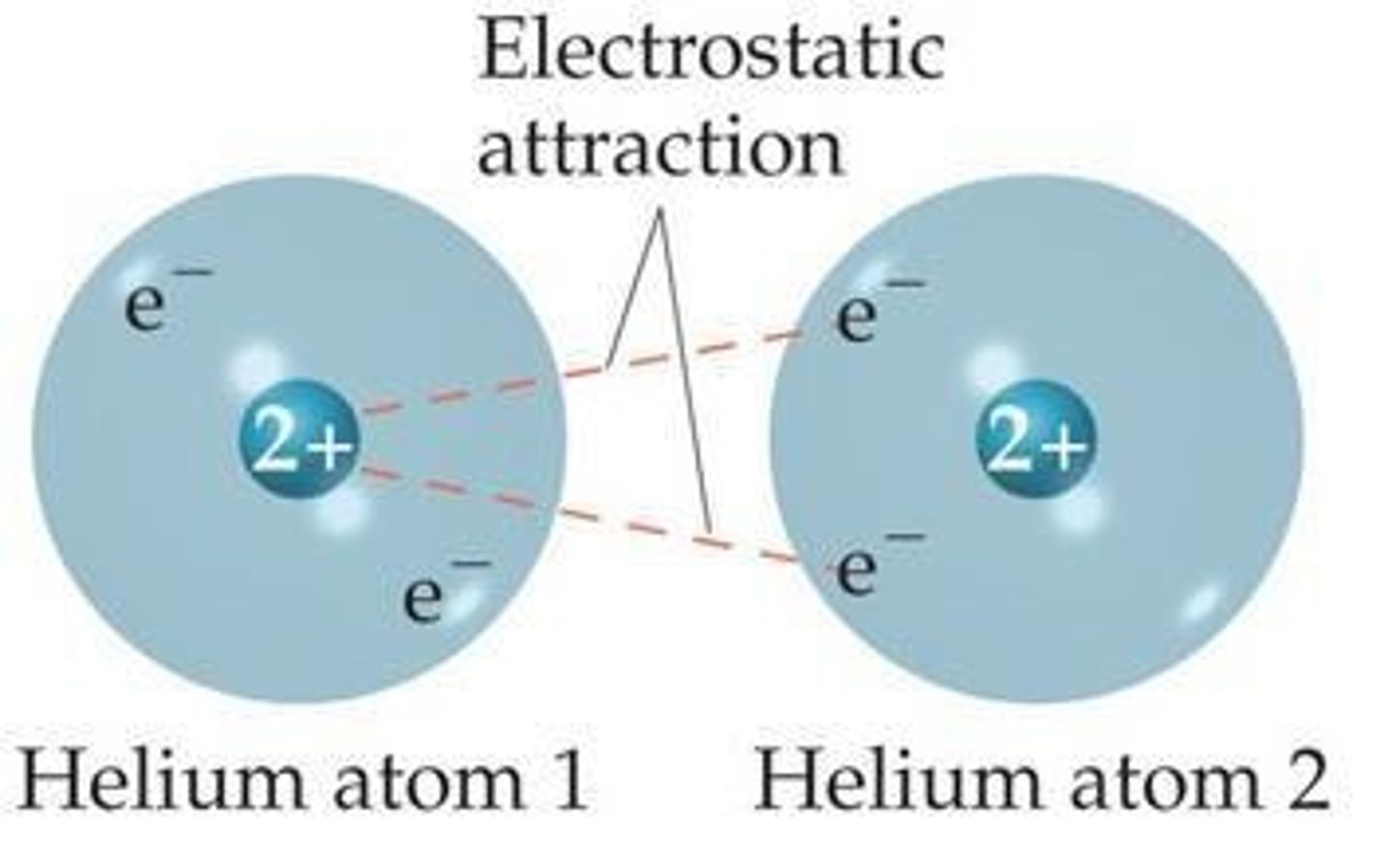
Strongest Intermolecular Force
Ion-ion interactions are the strongest intermolecular forces.
Electronegativity
The tendency of an atom to attract a bonding pair of electrons.
Physical Properties Influenced by IMFA
Boiling point, melting point, and solubility are influenced by the strength of intermolecular forces.
IMFA strength
Leads to higher boiling point, lower vapor pressure, stronger surface tension, and higher viscosity.
Crystalline solids
Have well-defined structures due to orderly arrangement of particles.
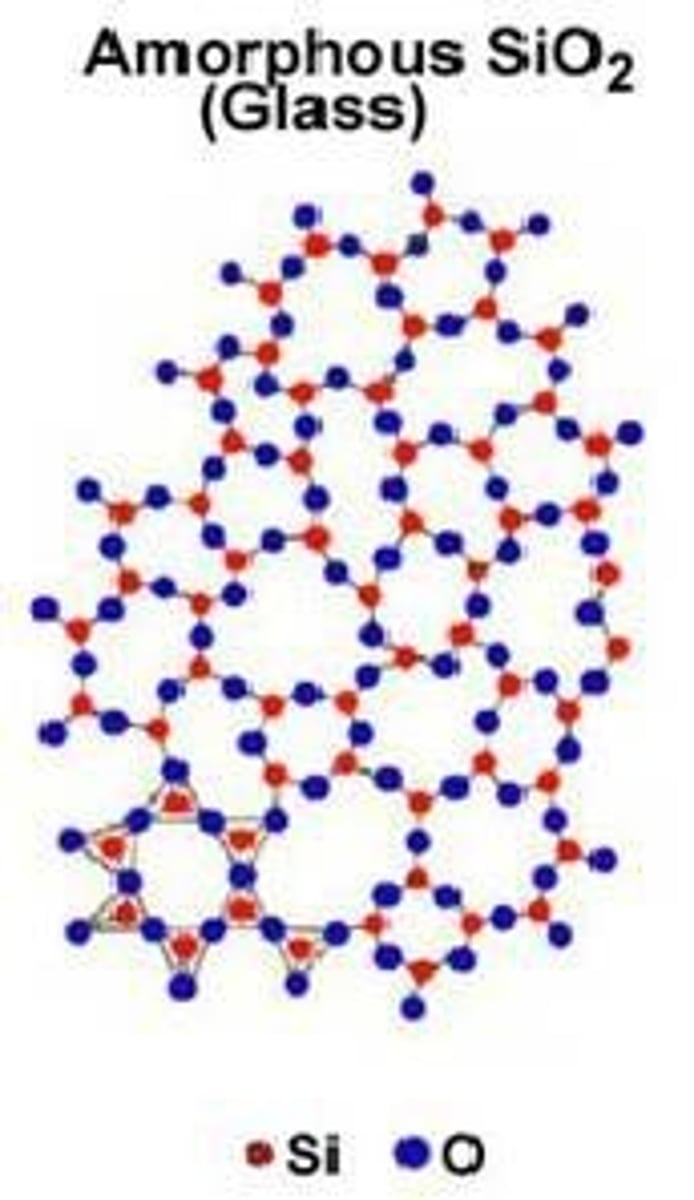
Amorphous solids
Do not have a well-ordered molecular structure; examples include waxes, glasses, and asphalt.
Molecular solids
Have molecules in the lattice positions, held by IMFAs, with low melting points and are volatile.
Ionic solids
Have ions in the unit cell, with high melting points, low conductivity, and are hard and brittle.
Network covalent solids
Linked by strong covalent bonds, with extremely high melting and boiling points.
Metallic solids
Held together by metallic bonding, with high electrical and thermal conductivity.