VSEPR
1/20
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
AB
Molecular shape: Linear | Electron geometry: Linear | Bonded atoms: 1 | Lone pairs: 0 | Bond angle: 180° | Example: H₂

AB2
Molecular shape: Linear | Electron geometry: Linear | Bonded atoms: 2 | Lone pairs: 0 | Bond angle: 180° | Example: CO₂

ABE
Molecular shape: Linear | Electron geometry: Linear | Bonded atoms: 1 | Lone pairs: 1 | Bond angle: 180° | Example: CN⁻
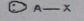
AB3
Molecular shape: Trigonal planar | Electron geometry: Trigonal planar | Bonded atoms: 3 | Lone pairs: 0 | Bond angle: 120° | Example: AlBr3
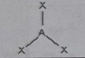
AB2E
Molecular shape: Bent | Electron geometry: Trigonal planar | Bonded atoms: 2 | Lone pairs: 1 | Bond angle: 120° | Example: SnCl₂
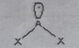
ABE2
Molecular shape: linear | Electron geometry: Trigonal planar | Bonded atoms: 1| Lone pairs: 2 | Bond angle: 120° | Example: O₂
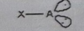
AB4
Molecular shape: Tetrahedral | Electron geometry: Tetrahedral | Bonded atoms: 4 | Lone pairs: 0 | Bond angle: 109.5° | Example: SiCl4
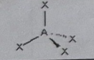
AB3E
Molecular shape: Trigonal pyramid | Electron geometry: Tetrahedral | Bonded atoms: 3 | Lone pairs: 1 | Bond angle: 109.5°| Example: PH₃
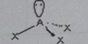
AB2E2
Molecular shape: Bent | Electron geometry: Tetrahedral | Bonded atoms: 2 | Lone pairs: 2 | Bond angle: 109.5° | Example: SeBr2
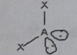
ABE3
Molecular shape: linear | Electron geometry: Tetrahedral | Bonded atoms: 1 | Lone pairs: 3 | Bond angle: 109.5°| Example: Cl2
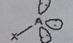
AB5
Molecular shape: Trigonal bipyramidal | Electron geometry: Trigonal bipyramidal | Bonded atoms: 5 | Lone pairs: 0 | Bond angle: 90° and 120° | Example: AsF₅
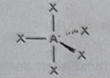
AB4E
Molecular shape: See-Saw | Electron geometry: Trigonal bipyramidal | Bonded atoms: 4 | Lone pairs: 1 | Bond angle: 90° and 120° | Example: SeH₄
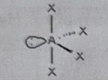
AB3E2
Molecular shape: T-shape | Electron geometry: Trigonal bipyramidal | Bonded atoms: 3 | Lone pairs: 2 | Bond angle: 90° and 120° | Example: ICl₃
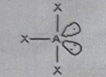
AB2E3
Molecular shape: Linear | Electron geometry: Trigonal bipyramidal | Bonded atoms: 2 | Lone pairs: 3 | Bond angle: 180° | Example: BrF₂⁻
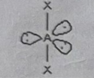
AB6
Molecular shape: Octahedral | Electron geometry: Octahedral | Bonded atoms: 6 | Lone pairs: 0 | Bond angle: 90° | Example: SeCl₆
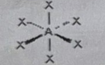
AB5E
Molecular shape: Square pyramidal | Electron geometry: Octahedral | Bonded atoms: 5 | Lone pairs: 1 | Bond angle: 90° | Example: IF₅
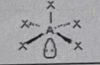
AB4E2
Molecular shape: Square planar | Electron geometry: Octahedral | Bonded atoms: 4 | Lone pairs: 2 | Bond angle: 90° | Example: XeF₄
ABE, ABE₃, ABE₄, ABE₅
There are no stable ABE, ABE₃, ABE₄, or ABE₅ molecules.
Bond representation
All bonds are represented as a line whether the bond is single, double, or triple.
Bonding domains
Any atom bonded to the center atom counts as one domain, even if bonded by a double or triple bond.
Domain count rule
The number of bonded atoms plus lone pairs always adds up to the total number of domains.