Ionic Bonding
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
When does ionic bonding occur?
when a metal & non-metal bond
What is ionic bonding?
the transfer of electrons that leads to strong electrostatic forces of attraction between oppositely charged ions
What is the charge of an ionic compound ?
neutral
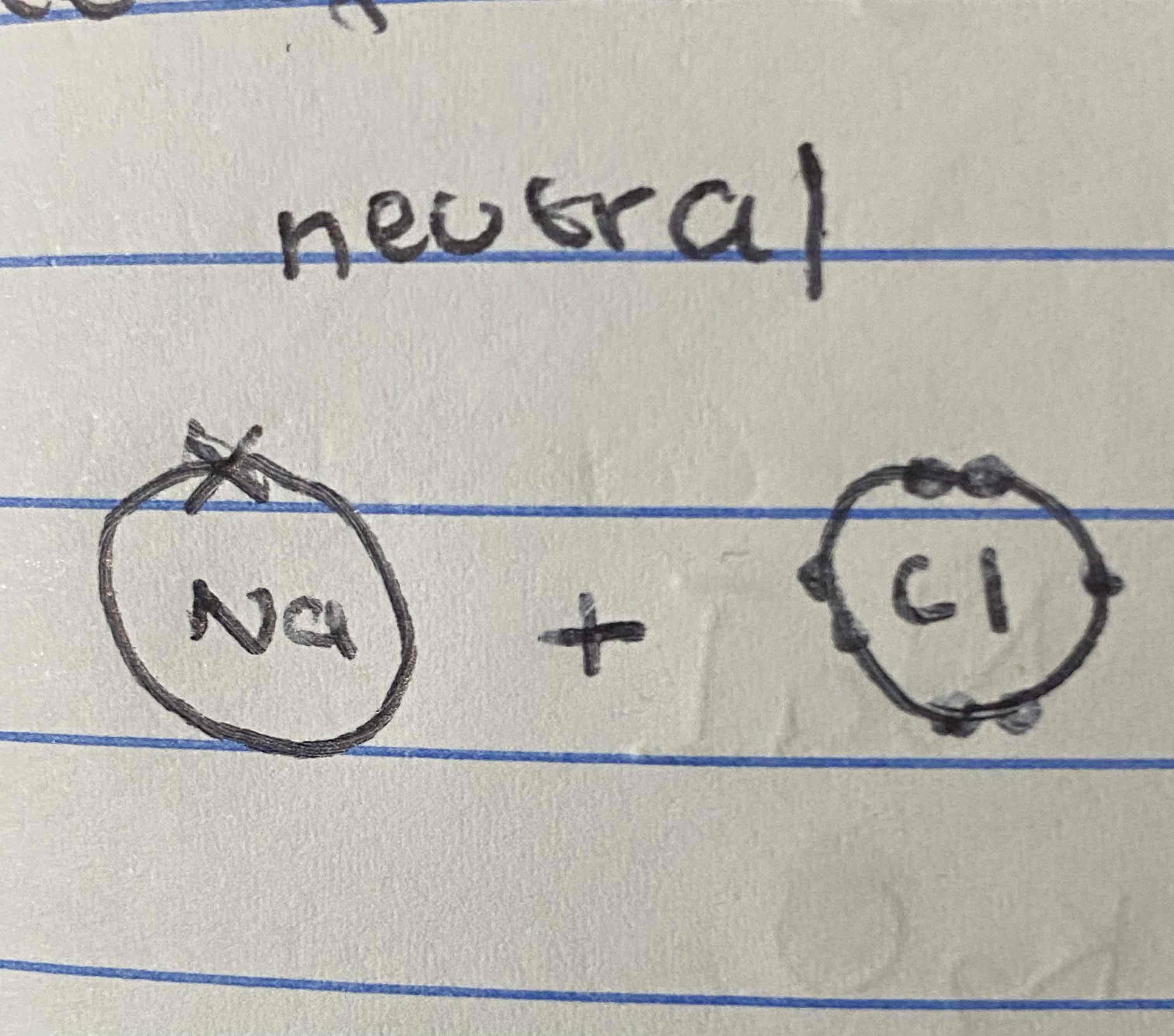
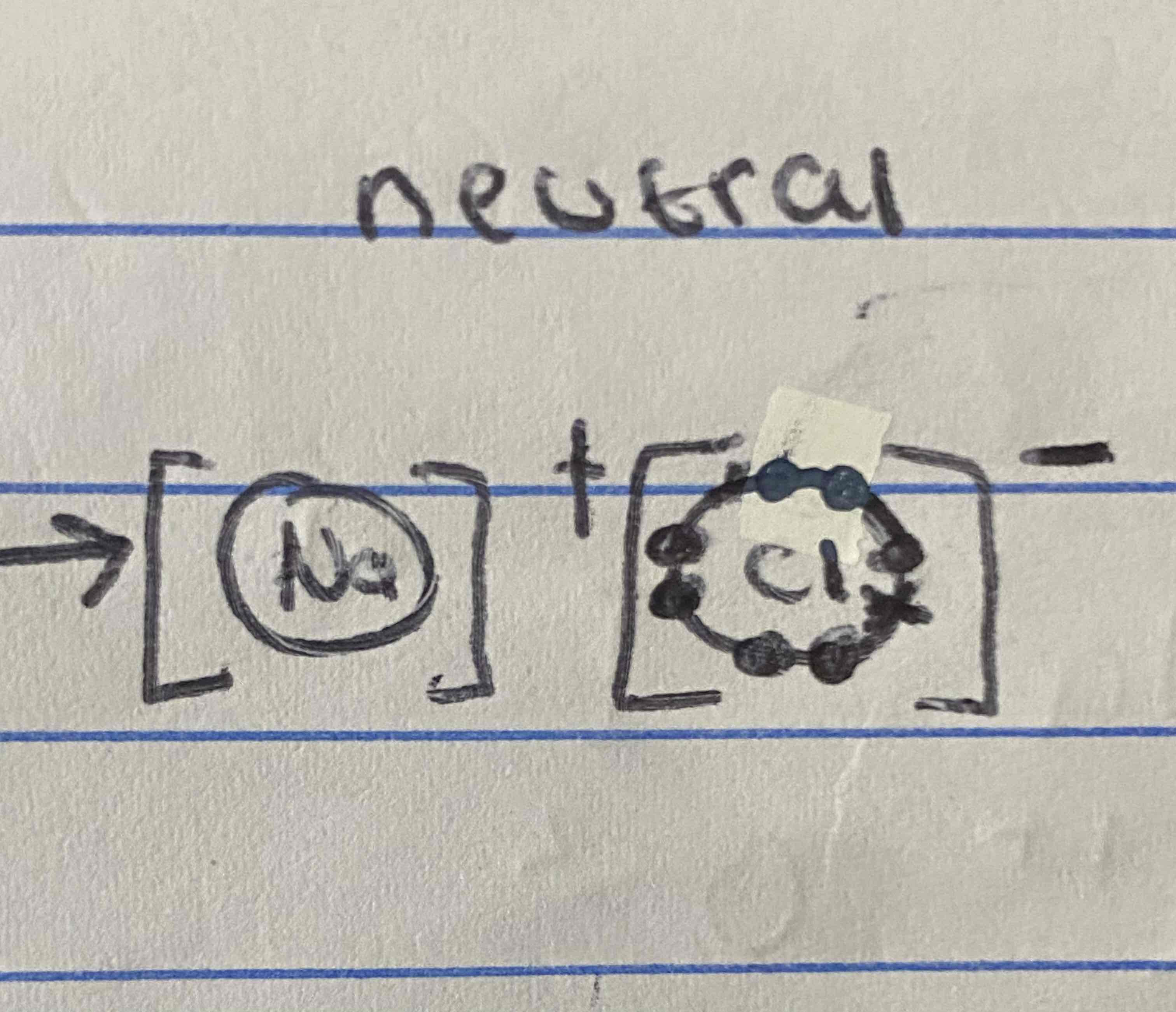
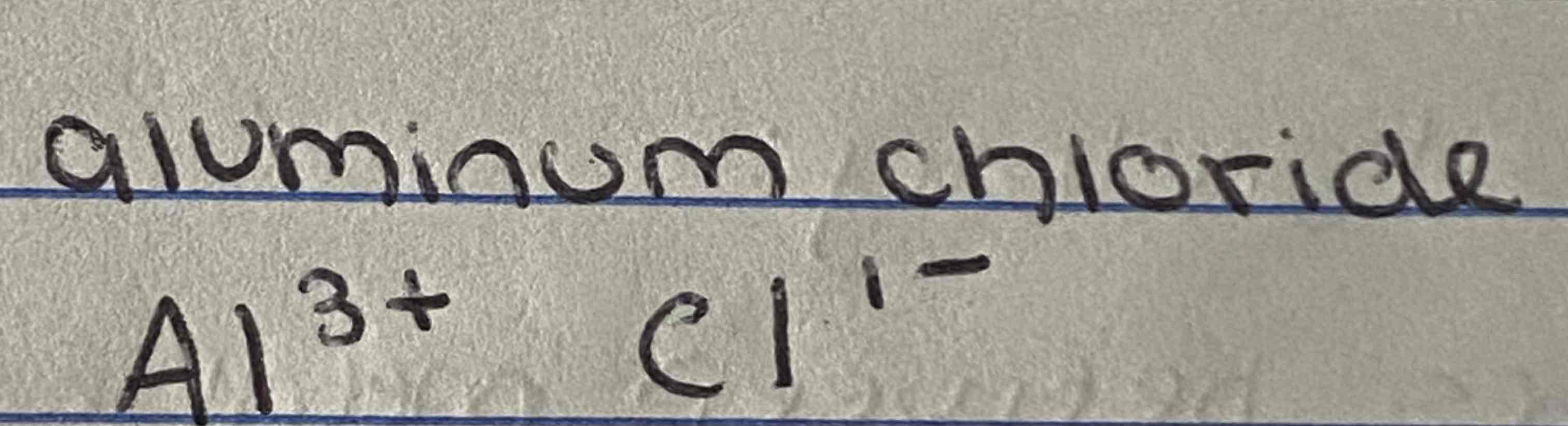
How would you write the formula for aluminium chloride using the swap and drop methond?
AlCl3
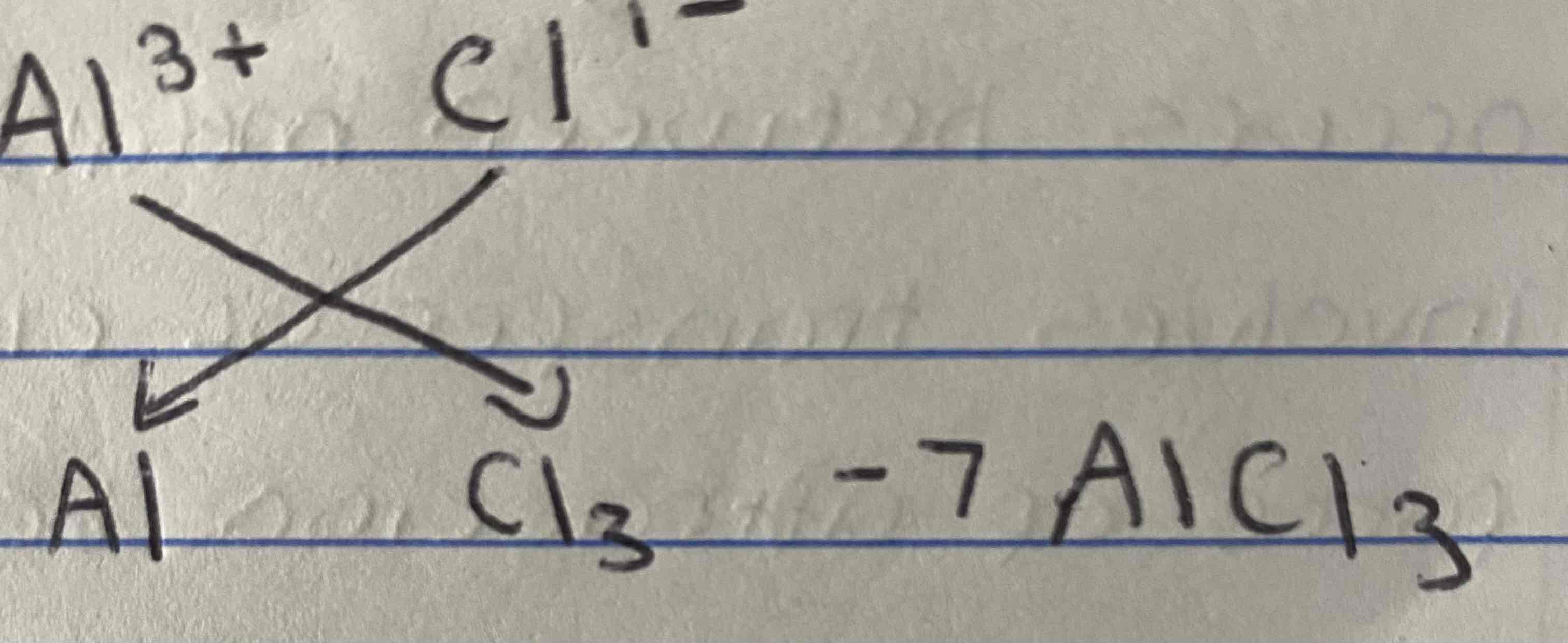
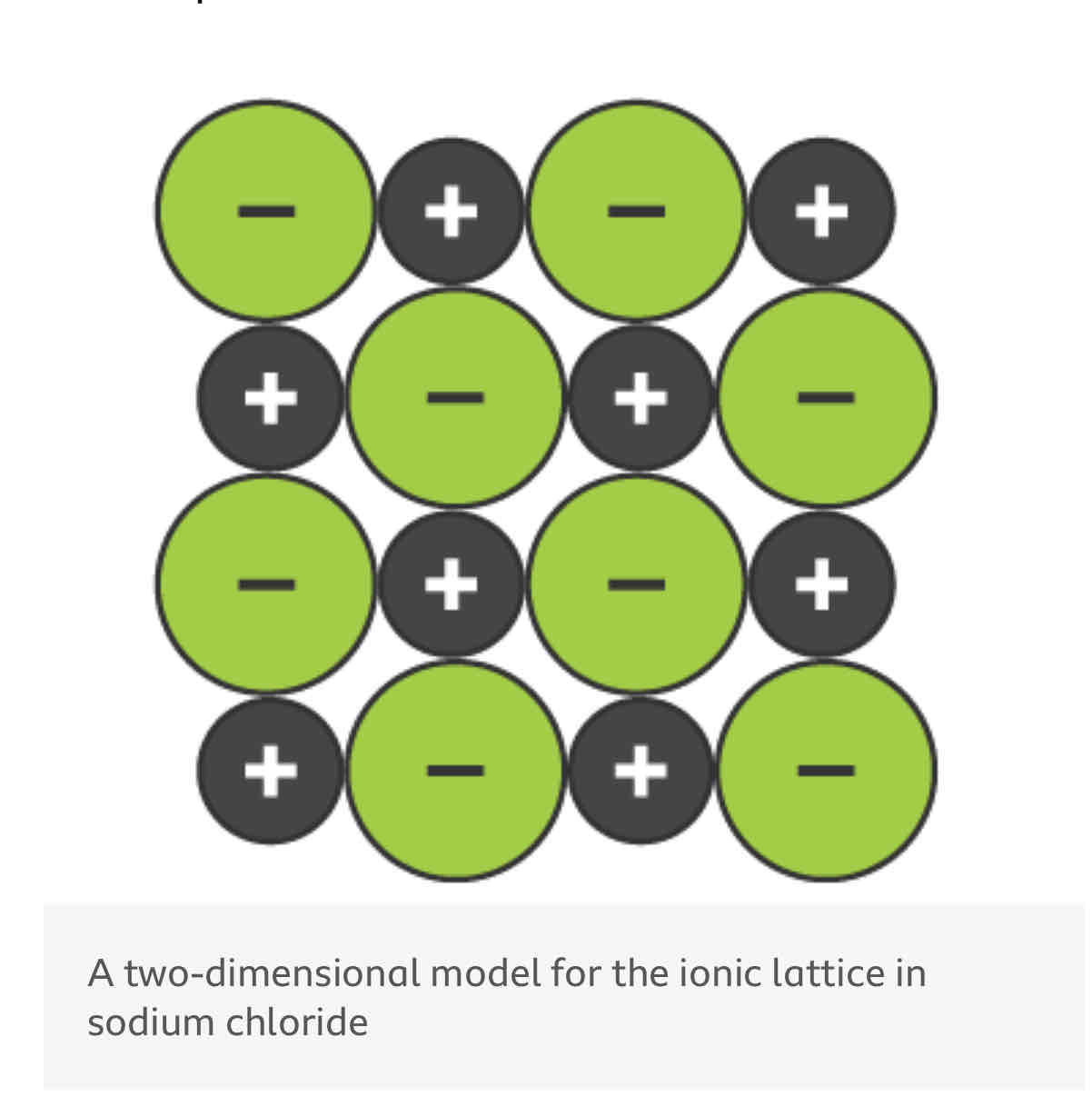
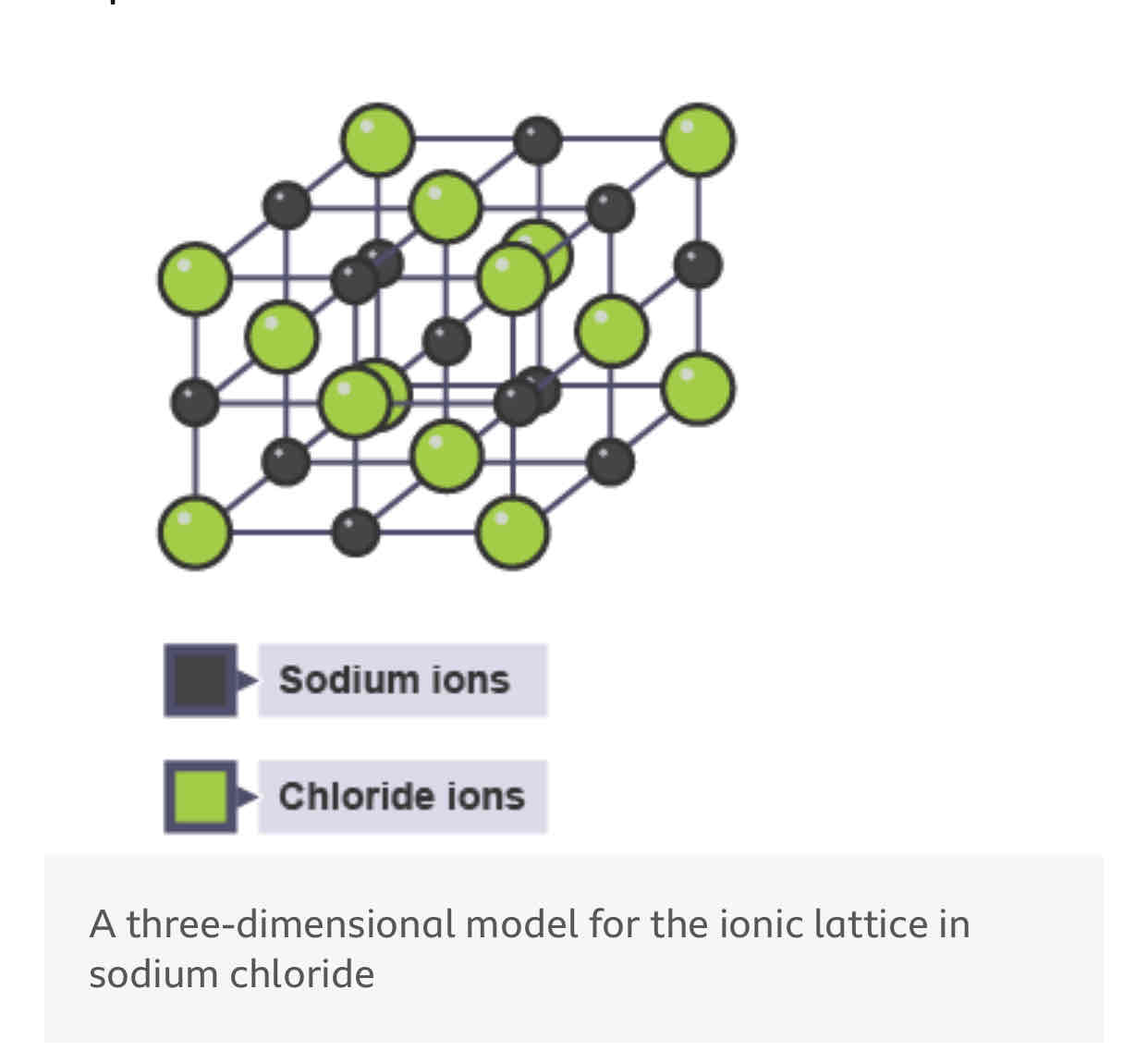
What is a giant ionic lattice?
in a regular arrangement repeated many times with a large number of ions
What are the properties of ionic compounds?
high melting/boiling point
can conduct electricity
brittle (layers move across each other)
they dissolve in water
Why can ionic structures dissolve in water?
water has a slight electrical charge so the can attract ions away from lattice
How do they conduct electricity?
they don’t when solid since ions are in a fixed position
when molten they conduct electricity as the ions are free to move around (mobile) and carry charge