Nucleas and Radioactivity
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Radioactivity
The breakup of unstable nuclei with the emission of one or more types of radiation.
Ionisation
Occurs when an atom loses or gains an electron. An ion is a charged atom.
What is an alpha particle?
The same as a Helium nucleus, it contains 2 protons and 2 neutrons and has +2 charge.
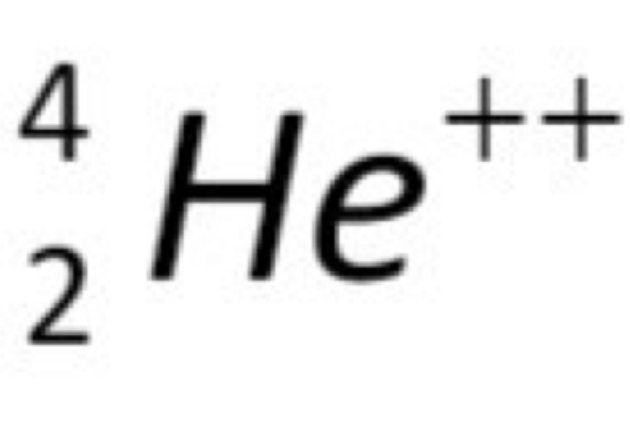
What is a Beta particle?
In beta decay, a neutron splits into a proton, an electron, and a particle called the neutrino. The Beta particle is the electron, which is fast moving and has a charge of -1.
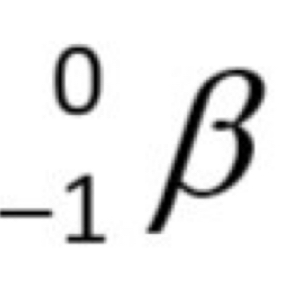
Gamma Radiation
A type of electromagnetic wave with a very short wavelength, meaning it has high frequency and high energy (given by E=hf)
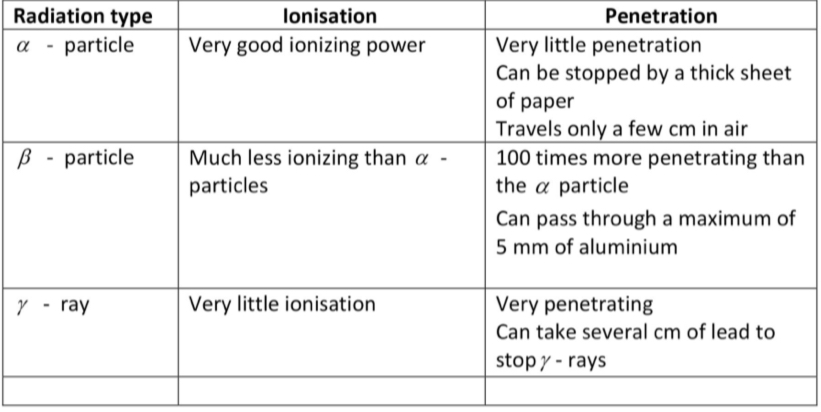
Comparison between the different particles and their ionisation and penetration.
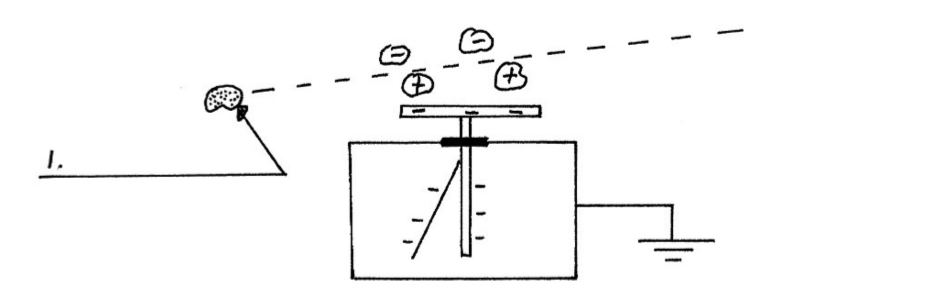
Demonstration Experiment for Ionization:
Charge a gold leaf electroscope. The gold leaf will be deflected away from the metal rod.
Bring a radioactive source near the cap.
The air just above the cap becomes ionized and some of these ions are attracted to the cap and neutralize some of the charges on the gold leaf electroscope.
The gold leaf is now less deflected.
Continual ionization of the air above the cap results in the total collapse of the gold leaf.
Since an alpha emitter is a better ionizer than the other radioactive sources the gold leaf falls more quickly if an alpha emitter is brought near the cap.
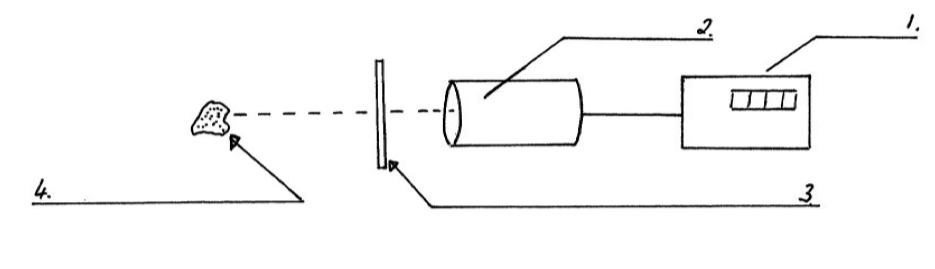
Demonstration Experiment for Penetration:
Bring an alpha emitter near the Geiger Muller tube and you will notice an increase in the count rate. Moving the alpha emitter more than 10 cm away and the count rate will drop. Placing a piece of paper in front of the alpha emitter and the count rate drops.
Bring a beta emitter near the G M tube and you will notice an increase in the count rate.
Moving the beta emitter away will make no difference unless the distance is quite far.
Placing sheets of aluminium in front of the beta emitter will result in a drop in the count rate. The beta particles cannot penetrate the aluminium.Bring a gamma emitter near the G M tube and you will notice an increase in the count rate. You will need to place sheets of lead a few cm thick in front of the gamma source to absorb the radiation, in order to notice a drop in the count rate.
Structure of an Atom

Rutherford/Geiger Marston gold foil experiment
a -particles were used to bombard a very thin piece of gold foil.
Most a -particles passed straight through the gold foil, some were deflected through a small angle and a very small number were deflected through angles greater than 90°.
The experiment was performed in a vacuum as a -particles have a very short range in air.
The a -particles were detected after hitting a fluorescent screen and causing scintillations i.e. small flashes of light.
Note(extra):
The nucleus has a positive charge and if an a -particle which is also positive passes near a nucleus it would be deflected.
If an a -particle was heading straight for a nucleus then it would be deflected through an angle greater than 90°.
As the nucleus is very small the probability if this happening is very low which explains why only a very small number of a -particles were deflected through angles greater than
90°.The size of the nucleus is about 1x10-15 m while the size of the atom is about
1x10-10 m.

Half Life (T_1/2)of an element
The time taken for half the radioactive nuclei in the sample to decay.
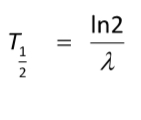
Isotopes
Atoms which have the same Atomic Number but a different Mass Number.
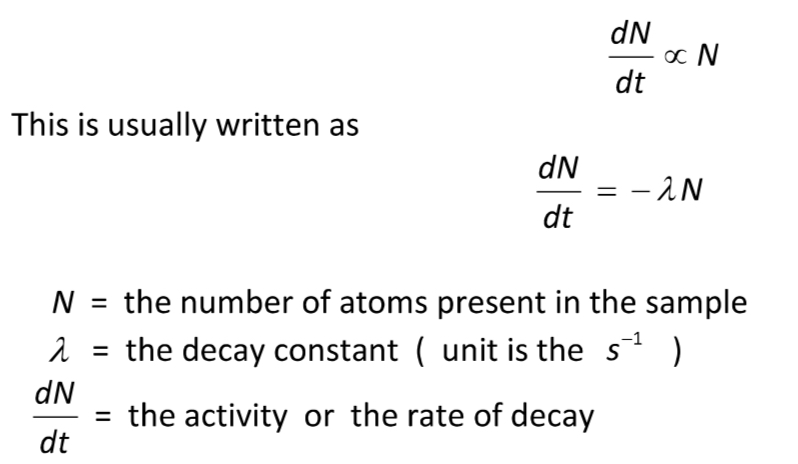
Law of radioactive decay
The rate of decay of a radioactive isotope is proportional to the number of atoms of the isotope present.
The Becquerel (Bq)
1 Bq is equal to 1 radioactive disintegration per second.
Precautions when dealing with ionising radiation
Make sure source is properly shielded.
Keep sources as far as possible from human contact.
Use protective clothing.
The effect of ionising radiation on humans depends on:
The type of radiation
Activity of the source
Time of exposure
Type of tissue irradiated.
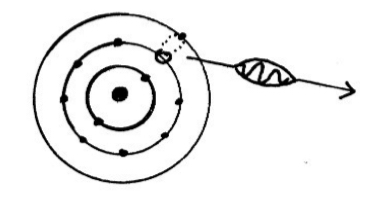
Bohr explanation of the atom
Bohr model of the atom:
Electrons only move in allowed orbits
If an electron moves to a higher energy level the atom is in an excited state If the electron returns to its lower energy level a photon of energy is emitted.The energy of the photon is given by
h f = E2 - E1
The energy of the photon is equal to the difference in the two energy levels.
Emission spectra - Line and continuous
When a gas absorbs energy, its electrons jump to higher levels.
As electrons return to their original levels, they emit photons of light with energy given by E = hf.
This produces an emission spectrum which is unique to each element.
A line emission spectrum
Emission spectra formed by gas.
Continuous emission spectrum
Created from an incandescent solid or liquid.