CHEM 2122 / Spectroscopy
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
Explain how a mass spectrometer separate and detect ions based on their mass-to-charge ratio (m/z).
> Molecules are ionized and fragmented.
> Send fragments through curved tube.
> Each fragment = Different formal charge = Different mass-to-charge ratio.
> Different curved path with specific m/z ratios to reach the detector.
> If uniform charge = m/z ratio is approximately the molecular mass.
+ Adjusting the magnetic field changes how much ions bend.
What does the height of the peak tell you in a mass spectrum?
What is the base peak in a mass spectrum?
What does the peak with the largest m/z ratio tell you?
• Height = Relative abundance of fragment.
• Base peak = Most abundant stable fragment = Most abundant ion.
• Peak w/ largest m/z = Molecular ion (M⁺) = Molar mass of entire molecule.
+ Stable fragments are less likely to break apart further, so more of them reach the detector, creating the tallest peak.
What does mass spectroscopy do for structure elucidation?
g/mol.
How does broadband infrared radiation interact with molecular bonds, and how is the absorbed energy detected?
What are the different vibrational modes available to a molecule?
Why do molecular vibrations occur at specific infrared frequencies?
What factors influence the absorption of infrared radiation by molecular bonds?
Some frequencies of energy from broadband infrared radiation is absorbed by specific bonds, which causes changes in the vibrational motions of a molecule, and the rest of the frequencies not absorbed is transmitted to a detector.
The different vibrational modes available to a molecule include stretching and bending modes.
The vibrational modes of a molecule are quantized, so the absorptions occur at specific energies which correspond to the frequency of infrared radiation.
The absorptions are dependent on the mass and strength of the bond.
In what units are IR absorption spectra calibrated?
Micrometers or in frequency-related wave numbers (cm-1), which are the reciprocals of wavelengths.
What is the relationship between transmittance and IR absorption?
In wavenumbers, what is the range that most bonds in organic molecules absorb energy?
What is the range of the functional group and fingerprint region on an IR spectrum?
What does the functional group and fingerprint region tell you?
IR absorption increases, transmittance decreases.
4000cm-1 to 400cm-1 is the range for absorption by organic molecles.
4000cm-1 to 1500cm-1 is the range for absorption of functional groups, with anything below 1500 being the fingerprint region.
The functional group region gives you easily deducible parts of the molecule, while the fingerprint region gives you not-so easily deducible parts of a molecule but is still characteristic to a molecule.
NOTE: Functional group regions are usually the “facial features” of a molecule, while the fingerprint regions are the “fingerprints” of a molecule.
In regards to polarity and symmetry, what type of bonds are usually associated with strong IR absorption? What bonds usually don’t accept IR absorption?
Polar and asymmetrical bonds are associated with strong IR absorption while symmetrical bonds with no dipole moment may not absorb at all.
Shorter and stronger bonds vs. longer and weaker bonds… where can you find each on the IR spectrum?
Bonds to lighter atoms vs. bonds to heavier atoms…. where can you find each on the IR spectrum?
Shorter and stronger bonds have their stretching vibrations at the higher energy end of the IR spectrum than the longer and weaker bonds, which are conversely at the lower energy end.
Bonds to lighter atoms vibrate at higher energy than bonds between heavier atoms.
How can you distinguish -NH stretches from -OH stretches?
How do you distinguish the stretches between primary amines and amides vs. secondary amines and amides?
What can tell you if you can coincidentally get a carboxylic acid? What can tell if you have an amide?
How can you differentiate O-H stretches from O-H stretches from a carboxylic acid?
-NH stretches are typically sharper and less intense.
Primary usually gives a “doublet,” while secondary usually gives a “singlet.”
You could get a carboxylic acid if you have an O-H stretch and C=O stretch. Likewise, you could get an amide if you have a N-H stretch and C=O stretch.
You can indicate an OH on a carboxylic acid when you have a broader and weaker OH stetch.
What is characteristic of an absorption of C≡C and C≡N in regards to their sharpness and strength?
What is characteristic of an absorption of C=O in regards to their strength?
These triple bonds are typically sharp but can be weak, particularly for internal alkynes where dipole moment is small.
These carbonyl bonds are often the strongest in the spectrum.
What is ATR, and how does it work?
Attenuated total reflectance, or ATR, is an IR spectroscopy technique for liquids and solids without extensive prep. It requires a crystal where IR light is totally internally reflected (e.g. ZnSe, Ge, or diamond) and good contact between sample and crystal. A wave penetrates into the sample where absorption occurs, and the rest of the wave is transmitted into the detector.
What does IR spectroscopy do for structure elucidation?
Functional groups present in the molecule.
How do spinning charges relate to magnetism, and why can nuclei behave as magnets?
How do nuclear magnetic vectors align in the presence and absence of an external magnetic field?
How does energy absorption by protons lead to the creation of an NMR spectrum?
How does the strength of the external magnetic field affect the energy difference between spin states?
Spinning charges generate magnetic fields, and can thus be magnetic vectors. Nuclei are charged, so they are magnetic vectors and can behave as magnets.
In absence of an external B-field, nuclear magnetic vectors are randomly aligned. However, once it is applied, all nuclear magnetic vectors are aligned with or against the field. An energy difference between the vectors (B0) is present because of their alignment against (higher in energy) or with (lower in energy) the B-field.
Photons of varying frequencies are directed into the sample. Protons absorb the energy and transition from a low-energy spin state (with the magnetic field) to a high-energy spin state (against the magnetic field). A spectrum is then created, showing the different proton environments and their relative abundances, based on the energy required to flip their spins.
Depending on the external magnet used, there will be different frequencies of light to spin flip the nucleus of a proton. The higher the magnetic field strength, the bigger the ∆E between spin states.
What are the three important features of proton NMR that assist in structure elucidation?
Chemical shift.
Multiplicity.
Integration.
What is chemical shift?
What does chemical shift indicate?
What is the formula for chemical shift?
Chemical shift is the difference in resonance frequency of a proton compared to a reference standard with 0 ppm (usually TMS) due to its chemical environment.
It indicates how shielded or deshielded the proton is from the external magnetic field.
Chemical shift (δ) in ppm = chemical shift from Hz / spectrophotometer frequency in MHz
Explain how chemical shift related to spin flipping via shielding/deshielding.
When a proton is near electronegative atoms (like in a carbonyl group), those atoms pull electron density away, reducing the shielding effect. This allows the external magnetic field to affect the proton more strongly.
With less shielding, the proton feels a stronger magnetic field, which increases the energy required to flip its spin (because the energy gap between the spin states is larger).
The higher the energy required to spin flip, the higher the chemical shift.
What determines whether or not a proton(s) will produce a single NMR signal?
Protons that are chemically equivalent (experiencing the same local magnetic environment) produce a single NMR signal.
What is spin-spin coupling? What does it do to NMR signals?
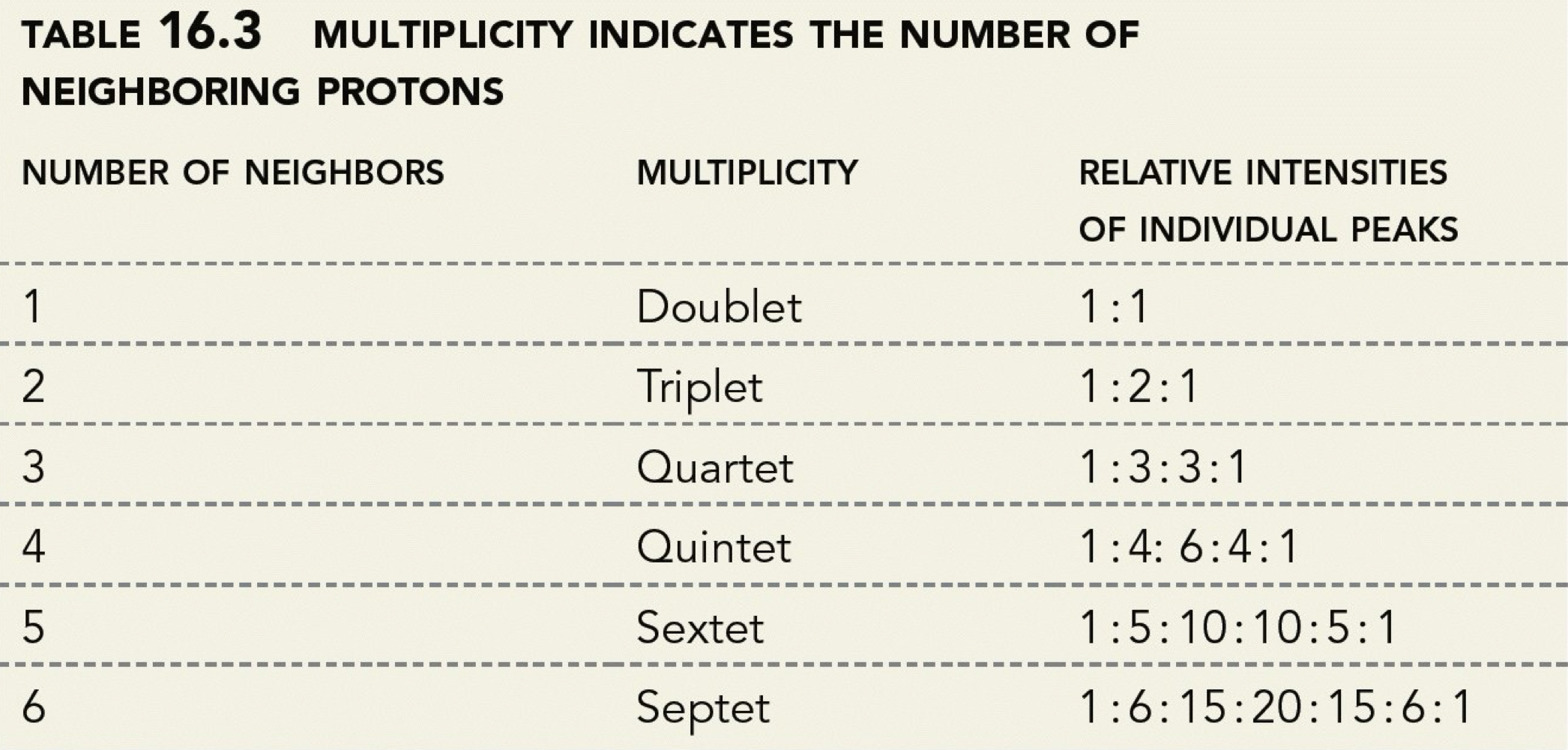
What is multiplicity? What math rule determines multiplicity?
Spin-spin coupling: Interaction between neighbouring, non-equivalent protons, causing their magnetic fields to influence each other. This splits NMR signals into multiple peaks.
Multiplicity: The number of peaks a signal splits into due to spin-spin coupling. It's determined by the n+1 rule, where n is the number of neighbouring protons.
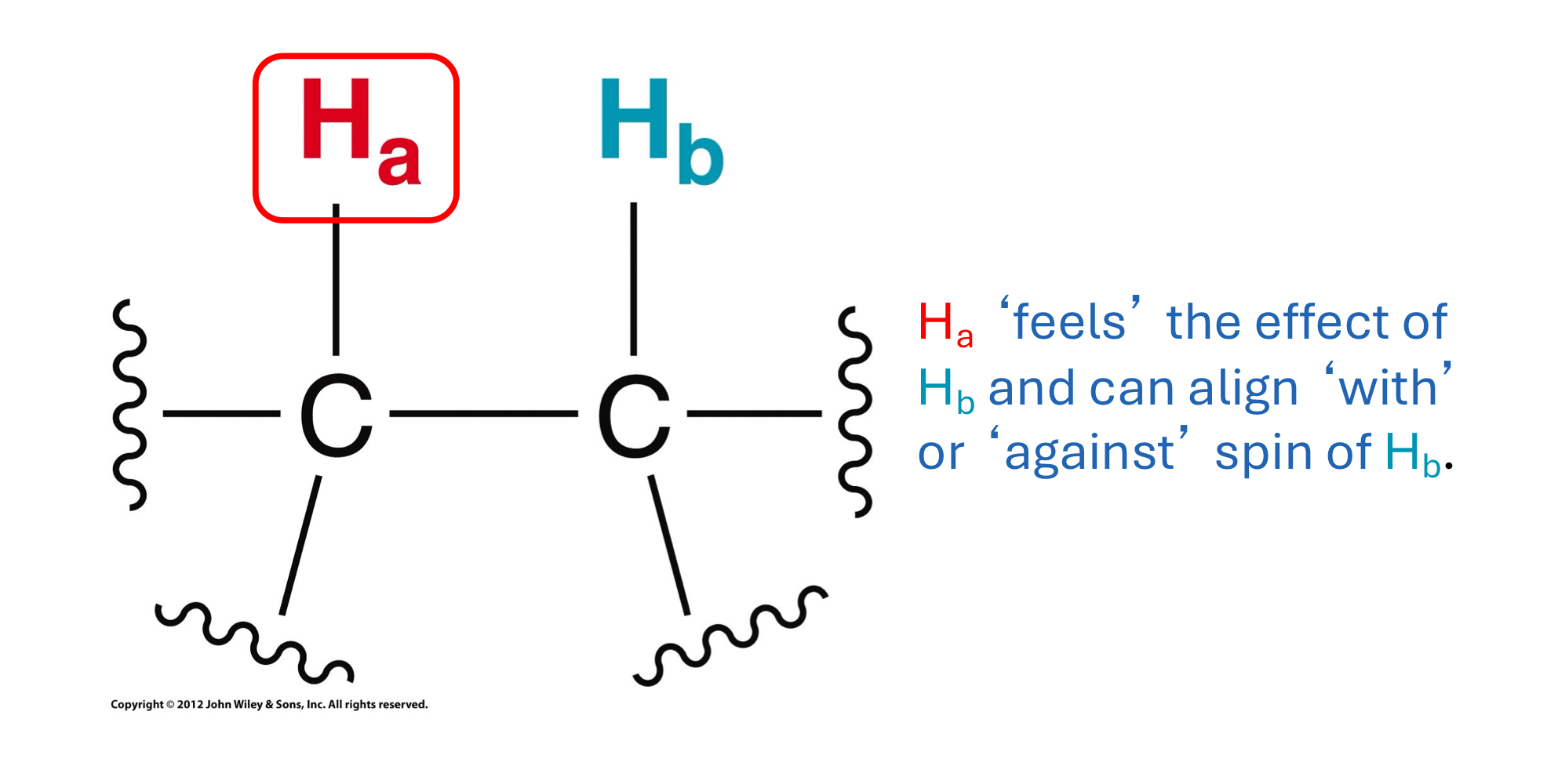
What are the first common six multiplicities corresponding to the number of neighbours? What are the relative intensities of their peaks?
Rank the deshielding of these neighbours towards protons: an electronegative atom, oxygen of an ester, and a carbonyl group.
Electronegative atom (highest shift).
Oxygen of ester.
Carbonyl group (lowest shift).
What are exchangeable protons, and where are they found, structurally?
How does deuterium exchange affect proton NMR signals?
Why do protons replaced by deuterium disappear from the NMR spectrum?
Exchangeable protons are those directly attached to electronegative atoms.
If deuterium is present in the solution, it can replace these protons through exchange.
Since deuterium doesn’t produce signals in proton NMR, the proton’s signal disappears from the spectrum.
What is integration?
Integration is the measurement of the area under each signal, which corresponds to the relative number of protons contributing to that signal.
What’s the difference between enantiotopic protons and diastereotopic protons?
Enantiotopic protons are protons that are chemically equivalent in a non-chiral environment.
Diastereotopic protons are protons that are not equivalent in a chiral environment.
What is the influence of chirality on NMR signals?
Chiral centers create non-equivalent proton environments because they cause asymmetry in the molecule. The spatial arrangement around a chiral center makes the protons attached to neighboring atoms experience different electronic and steric effects. As a result, even protons that are in similar positions in the molecule can feel slightly different magnetic environments, leading to distinct NMR signals.
What is the effect of the following neighbours on chemical shift of an alpha proton?
What is the effect on beta protons?
Oxygen of an alcohol or ether.
Oxygen of an ester.
Carbonyl groups.
Oxygen of an alcohol or ether: +2.5 ppm.
Oxygen of an ester: +3.0 ppm.
Carbonyl groups: +1.0 ppm.
The effect of beta protons is generally about 1/5 of the effect on alpha protons.
What’s the important difference between proton NMR and carbon (13C) NMR in regard to chemical shift, multiplicity, and integration?
Chemical shift: The shift still tells you about the environment of carbon but is now determined by electronegativity and hybridization, as carbon can hybridize, while hydrogen cannot.
Multiplicity: All peaks are singlets due to broadband decoupling, which decouples the ½ spin of protons interacting with the ½ spins of carbons. No coupling occurs between 13C and 13C because of the low abundance.
Integration: Because of the low abundance of carbon-13, integration is not reliable and NMR is now not quantitative. However, if the carbon is attached to a hydrogen, the larger the intensity of the peak. If the carbon is not attached to a hydrogen, the lower the intensity of the peak.
What is the chemical shift of CDCl3? What do we do with this during analysis?
Above what shift will you usually find sp2 hybridized carbons? Above what shift will you usually find sp3 hybridized carbons?
77.0 ppm. This is present in all of the carbon-13 NMR spectra, but it can be ignored.
Signals above 100 ppm are sp2 carbons, while signals below 100 ppm are sp3 carbons.
What does DEPT do in carbon-13 NMR, and how does it help in analysis?
DEPT (Distortionless Enhancement by Polarization Transfer) is a technique used in carbon-13 NMR to enhance the signals of quaternary, methine, methylene, and methyl carbons. It helps to differentiate between different types of carbon atoms based on the number of attached protons.
What does DEPT-90 tell you?
What does DEPT-135 do?
DEPT-90: Shows only methine (CH) carbons, giving a positive peak for each CH group.
DEPT-135: Shows methyl (CH₃) and methine (CH) carbons as positive peaks, while methylene (CH₂) carbons appear as negative peaks.
What is the purpose of COSY, and how does it help in analysis?
Purpose: Shows proton-proton (¹H-¹H) correlations, showing how protons are coupled through bonds, showing which protons are connected through 2-3 bonds (usually).
Help: Helps map out proton connectivity in a molecule.
What is the purpose of HSQC, and how does it help in analysis?
Shows [1J] correlations between protons (¹H) and directly bonded carbons (¹³C). It allows you to see which protons are attached to which carbons.
What is the purpose of HMBC, and how does it help in analysis?
Correlates [2J and 3J] protons (¹H) with carbons (¹³C) that are 2-3 bonds away, allowing you to see longer-range proton-carbon relationships.
What’s a good rule of thumb in HMBC J couplings with aromatic/conjugated and aliphatic systems.
3J couplings are almost always stronger for aromatic/conjugated.
2J coupling are almost always stronger for aliphatic.
What’s the best way to solve spectral problems with all spec techniques learned?
Mass spec: Get the formula.
IR spec: Get the functional groups.
Carbon-13 NMR: Get the number of carbons and types of carbons.
Proton NMR: Elucidate the chemical environment.
Mass spec: Confirm with mass and formula.
What’s the formula for index of hydrogen deficiency?
What does the index tell you.
What does a high index indicate?
Index = # of carbons - (# of hydrogens/2) - (# of halogens/2) + (# of nitrogens/2) + 1
Index tells you “how many” double bonds there are.
High index of 4 or higher suggests aromatic ring.
What is the nitrogen rule?
If the molecular ion from mass spec has an odd MW, there must be an odd number of nitrogens in a molecule.
Provide the approximate IR absorption of the following. Provide broadness and strength if applicable.
C-H.
O-H.
N-H.
C=O.
C≡C.
RC≡C-H.
C≡N.
C-H: 2900cm-1 approximate. Broadness depends on nature of C-H environment. Very strong absorption.
O-H: 3500cm-1 approximate. Very broad and very strong absorption.
N-H: 3500–3000cm-1 approximate. Somewhat broad and strong absorption.
C=O: 1800–1600cm-1 approximate. Very sharp and very strong absorption.
C≡C: 2120cm-1 approximate.
RC≡C-H: 3100cm-1 approximate.
C≡N: 2200cm-1 approximate.
Provide the approximate chemical shift of the following.
R-CH3.
R2-CH2
R3-CH
R4-C.
R3-COH.
R2-C=C-R2.
C in benzene.
R2-C=O.
R-CH3: 10–30 ppm.
R2-CH2: 20–55 ppm
R3-CH: 30–60 ppm.
R4-C: 30–40 ppm.
R3-COH: 50–90 ppm.
R2-C=C-R2: 100–150 ppm.
C in benzene: 100–160 ppm.
R2-C=O: 160–220 ppm.
Provide the approximate chemical shift of the following.
C in amides.
C in esters.
C in carboxylic acids.
C in ketones.
C in aldehydes.
C in amides: 150–175 ppm.
C in esters: 150–175 ppm.
C in carboxylic acids: 165–180 ppm.
C in ketones: 185–210 ppm.
C in aldehydes: 200–220 ppm.