Electrode potential and electrochemical cells
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
What is a half cell and the types
An electrode used to establish how much reduction or oxidation os occuring
A metal electrode - A metal is placed in a soln containing its ions
Gas electrodes- Inert metal. gas and soln
Redox electrodes- Inert metal and two different aqueous ions
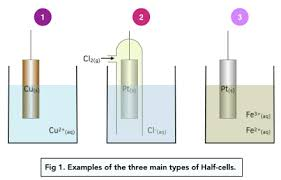
What is the standard electrode potential and necessary conditions
It is the potential difference/ voltage between two half cells relative to SHE having a value of 0.00v under standard conditions.
1moldm3 of relevant ions
298k
100KPa (when gases are involved)
What is an electrochemical cell
Two half-cells joined together which can power a component. The voltage can be measured by replacing the component with a voltmeter.
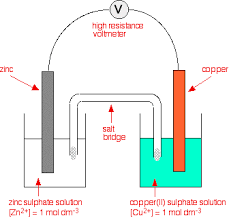
Draw a gas/metal electrode and why is platinum required
It is an inert metal that provides a surface for electron transfer
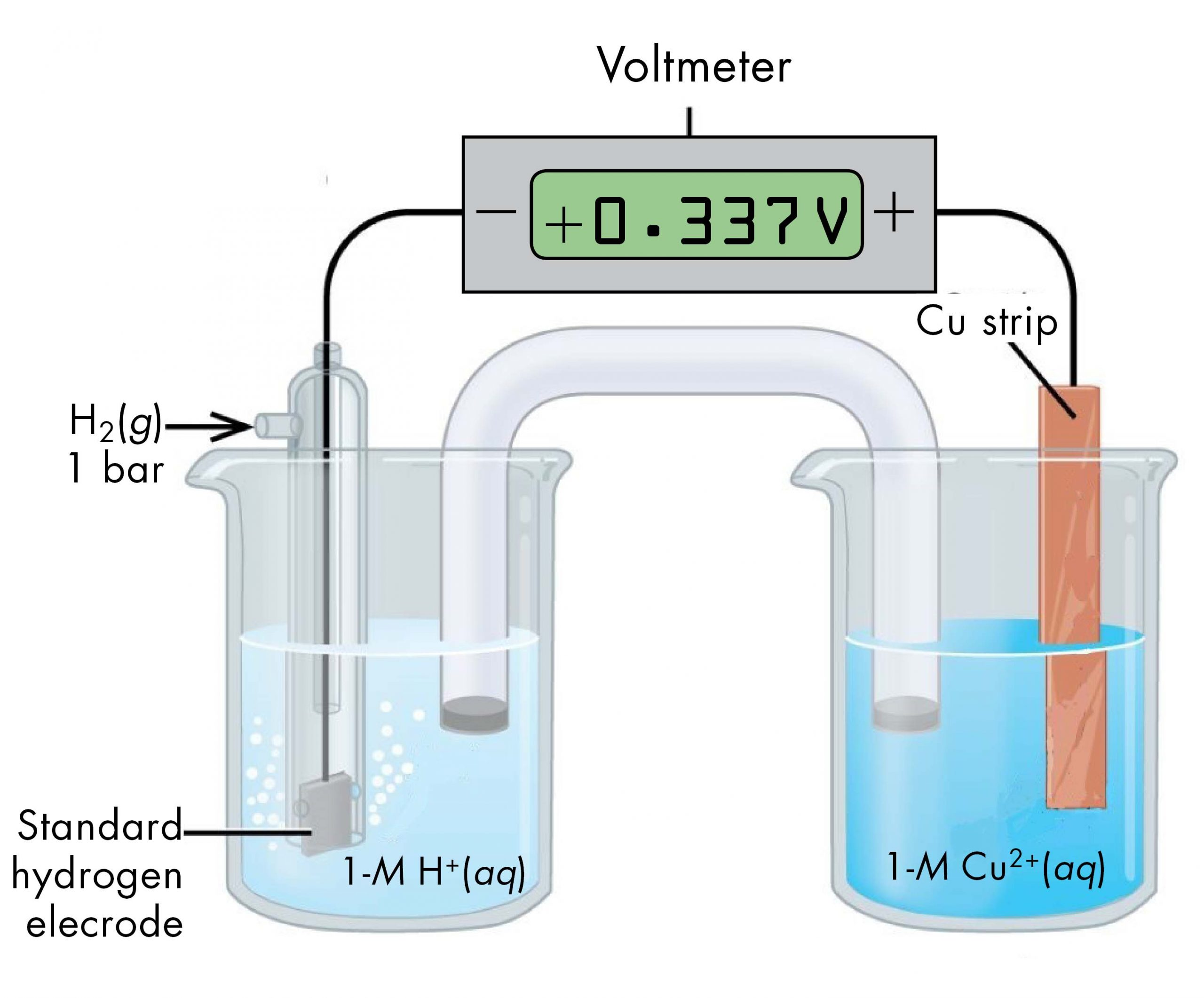
Draw a gas/redox electrode

2 redox electrodes
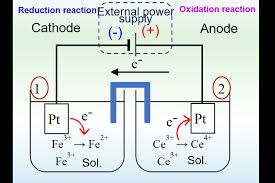
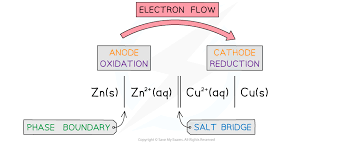
What is the conventional cell representation and examples
The LHS is the anode the double line is the salt bridge, the RHS is the cathode and the vertical lines represent phase boundaries
The hydrogen electrode if involved is always on the LHS and the species with the highest oxidation state is always nearest to the salt bridge. A comma us used to seperate different species of the same phase
aq and l are the same phase
The more -ve always goes on the left except for qhen SHE is presenr
If H2O is involved( but not the main reactant and product) H+ always goes in the middle of the species NOT H20
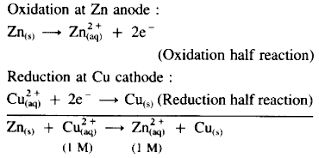
Calculating electrode potential
E= Ered - Eoxi
ie ERHS -ELHS
How is an overall reaction written for an electrochemical cell
Since all reactions in an electrochemical series are written as reduction reactions, the half reaction with the most negative value is flipped to be an oxidation reaction.
NB: The E values are given in the question
How does eqbm affect the overall E value for a reaction in an electrochemical cell
If eqbm shifts right, E value increases ie becomes more +ve
If eqbm shifts left E value decreases ie becomes more -ve
NB: Le Chatelier’s principle also applies
What does the E value represent in any electrochemical series
How good the oxidising agent is at oxidising a R.A or how good a reducing agent is at reducing a R.A
What are good O.A
The species on the left of any electrochemical series is the O.A and the reaction with the most +ve E value is the best O.A.
Therefore its is more likely to be reduced as the forward reaction is more favourable.
What is a good R.A
The species on the right of any electrochemical series, and the reaction with the most -ve Evalue is the best R.A
Therefore it is more likely to be oxidised as the backward reaction is more favourable.
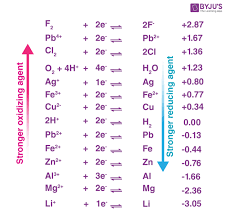
How is the feasibility of a reaction determined
A more +ve E value tells us the reaction is feasible and vice versa.
Will h+ oxidise Fe to Fe2+
E°(H+ /H2) > E°(Fe2+/Fe) and therefore H+ gains electrons from Fe
2H+ + Fe → H2 + Fe2+
Will Cr2072-/H+ oxidise Cl- to Cl2
E°(Cr2O7 2– /Cr3+) < E°( Cl2/ Cl – ) and therefore Cr2O7 2– cannot gain electrons from Cl –
What is a battery
2 or more cells connected in series
What are primary cells and examples
Non-rechargeable battery cells
Zinc- carbon (cheap+ runs out quickly)
Alkaline ( more expensive but lasts longer)
Overtime the conc of reactants decreases causing E value to drop to 0.00v
What is a secondary cell, advantages and examples
A battery that can be recharged. This happens because the external power source allows the reverse reaction to occur so the conc of reactants builds up again
Lead- acid eg car batteries
Fuel cells eg AA batteries
Adv: High power
Lower density
Disad: Expensive
Reacts with air and moisture
Reaction in Lithium batteries (secondary cell)
Li+ + CoO2 + e- —→ Li+ [CoO2 ] - E=+0.6V (cathode)
Li+ + e- ——>Li E=-3.0V (anode)
Overall reaction during discharge
Li + CoO2 ——> LiCoO2 E=3.6V
Overall reaction during recharge is the same as discharge but flipped.
Conventional cell rep
Li | Li+ || Li+ , CoO2 | LiCoO2 | Pt
NB: All reactions listed above are reversible not direct.
Water is not used as a solvent because lithium reacts violently with water
What are fuel cells, adv and disad
A secondary cell that requires a continuous supply of reactants
They involve H2, glucose, methanol/ethanol
adv: Only waste product is water
No GHG emissions
Limitless
More efficient
Disad: H2 is expensive and flammable
H2 must be stored as a liquid
H2 gas has to be made first which burns fossil fuels, so does emit GHG as CO2 is released
H2 fuel cell reactions at the anode and cathode
2H2 (g) + 4OH– (aq) → 4H2O (l) + 4e– Eꝋ = -0.83 V (anode)
O2 (g) + 2H2O + 4e– → 4OH– (aq) Eꝋ = +0.40 V (cathode)
overall reaction
2H2 (g) + O2 (g) → 2H2O (l) Eꝋ = +1.23 V