2CB3 Cell Bio Exam Review
5.0(1)
Card Sorting
1/147
Earn XP
Last updated 2:42 PM on 12/12/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
148 Terms
1
New cards
What are the basic properties of cells (Hint there are 9)?
1. cells are highly complex and organized
2. cells posses a genetic program and the means to use it
3. cells can produce more of themselves
4. cells acquire and use energy
5. cells carry out a variety of chemical reactions
6. cells engage in mechanical activities
7. cells are able to respond to stimuli
8. cells can self-regulate
9. cells can evolve
2
New cards
List 5 differences between eukaryotes and prokaryotes:
Eukaryotes:
- are multicellular and have membrane bound organelles
- has a nucleus and nuclear division and is separated by a nuclear envelope
- is a diploid so have two copies of genes
- have complex flagella and cilia
- has mitochondria and chloroplasts
Prokaryotes:
- unicellular and no membrane bound organelles
- has no nucleus
- is a haploid
- has simple flagella and cilia
- no mitochondria or chloroplasts
- are multicellular and have membrane bound organelles
- has a nucleus and nuclear division and is separated by a nuclear envelope
- is a diploid so have two copies of genes
- have complex flagella and cilia
- has mitochondria and chloroplasts
Prokaryotes:
- unicellular and no membrane bound organelles
- has no nucleus
- is a haploid
- has simple flagella and cilia
- no mitochondria or chloroplasts
3
New cards
What is a proteasome and what is it its function?
they are protein complexes responsible for the degradation of un-needed or damaged proteins by proteolysis (the breaking of peptide bond). enzymes that help this reaction are called proteases.
4
New cards
How many domains of classification are there and name them.
3, bacteria, archaea and eukarya
in Archaea there are thermophiles, acidophiles, methanogens and halophiles.
in Archaea there are thermophiles, acidophiles, methanogens and halophiles.
5
New cards
What are the characteristics of mycoplasma?
is a prokaryote that is the smallest and simplest. It has no cell wall and is thus resistant to antibiotics since antibiotics attack the cell wall.
6
New cards
What organism would you use for different experiments?
- E.coli us great for genetic manipulation and is rod shaped
- Yeast is least complex of eukaryotes used to study proteins homologous to human cells (metabolic, neurodegenerative)
- wheat is used for its short generation time, large seed production and height of a few inches
- C.elegans has a defined number of cells (1000), easy to control, precise pattern of cell division, transparent body walls and short generation time
- fruit fly turn into adults in 10 days
- house mouse are good for cancer and also due to their size
- Yeast is least complex of eukaryotes used to study proteins homologous to human cells (metabolic, neurodegenerative)
- wheat is used for its short generation time, large seed production and height of a few inches
- C.elegans has a defined number of cells (1000), easy to control, precise pattern of cell division, transparent body walls and short generation time
- fruit fly turn into adults in 10 days
- house mouse are good for cancer and also due to their size
7
New cards
What is a stem cell?
Its is an undifferentiated cell that can self-renew and is multi-potent so it can differentiate into 2+ mature cell types.
8
New cards
What is an Embryonic Stem (ES) cell?
Is a pluripotent stem cell but cannot turn into the placenta and umbilical cord. They can also self-renew in culture. They can turn into benign tumours known as teratomas.
9
New cards
Describe direct cell reprogramming:
When a differentiated cell is reprogrammed into another differentiated cell without undergoing the pluripotent state. Acinar cells from the pancreas are transformed into pancreatic beta cells (which produce human insulin) in diabetic mice. To do this 3 genes from a virus will be injected into the acinar differentiated cell and thus it will be involved in the differentiation of beta cells in diabetic mice. They are thus transdifferentiated into a beta cells.
10
New cards
A mitochondrion is 2 μm long. How many angstrom would it be?
20000 Å
11
New cards
What is a viroid?
- Small naked RNA molecule (no protein coat)
- Doesn't need to inject into host
- will interfere with gene expression in host
- Doesn't need to inject into host
- will interfere with gene expression in host
12
New cards
Is coronavirus a virion or viroid? Why?
Virion, has a coat
13
New cards
How do viruses infect?
Viruses have surface proteins that bind them to the surface of the host. They have viral specificity and will infect a hosts specific cells depending on its surface proteins.
14
New cards
What is a covalent bond?
It involves the sharing of electrons between two atoms
15
New cards
What is a polar bond?
Is where the sharing of electron is unequal and there is a partial charge present on each side of the molecule
16
New cards
What is a non-polar bond?
Electrons are equally shared between atoms.
17
New cards
What is a non-covalent bond?
Have attractions that are weaker than a covalent bond. Doesnt depend on electron sharing , Depends on attraction between opposite charges. On their own are weak bond, 1-5 kcal, that break and reform.
18
New cards
Why are non-covalent bonds important in cells?
Aids in dynamic cellular processes. Individually they are weak but collectively they can provide structural stability. Ex the 2 DNA strands
19
New cards
What is an ionic bond?
Is electrostatic force between fully charged atoms. Gets weaker in presence of water. Stronger in the core of a proteins.
Ex: Phosphate atoms, -ve charge, from DNA are closely associated with +ve charged groups on a protein
Ex: Phosphate atoms, -ve charge, from DNA are closely associated with +ve charged groups on a protein
20
New cards
What is a hydrogen bond?
When a covalently bonded hydrogen (+ve charge) of one molecule is attracted to the electrons of another molecules atom. Determines structures and properties of water. Ex: The bonds between the 2 stands of DNA
21
New cards
What are hydrophobic interaction and Van Der Waals Forces?
Intermolecular forces (forces between 2 separate molecules) between hydrophobic molecules and water. The hydrophobic molecules will form in a way that will limit their interaction with water. Is not a true bond. Significant within larger biological molecules (away from water). Due to dipoles. Sensitive to the distance between 2 atoms/molecules.
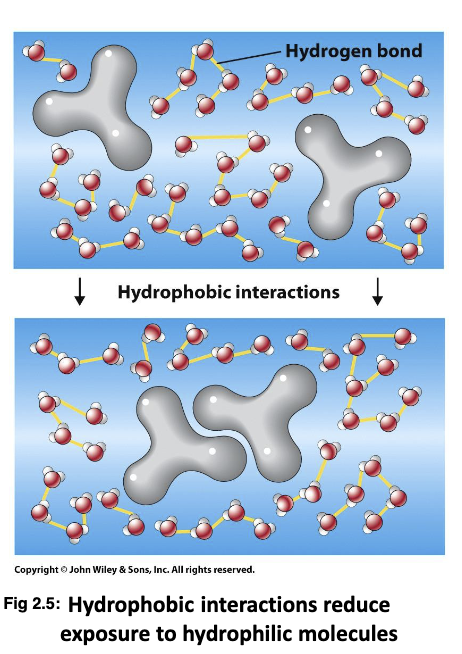
22
New cards
What are the 4 biological molecules?
- Carbohydrates
- Lipids
- Proteins (polymers of amino acids)
- Nucleic acids (polymers of nucleotides)
- Lipids
- Proteins (polymers of amino acids)
- Nucleic acids (polymers of nucleotides)
23
New cards
Describe carbohydrates:
Used for energy storage and structural support. Can be used for biochemical signature on cell surfaces. Participates in cell recognition and structural roles.
Chemical Formula: (CH2O)n
Chemical Formula: (CH2O)n
24
New cards
What is a ketose?
Sugar that has a carbonyl (C=O) on internal carbon
25
New cards
What is an aldose?
Sugars with carbonyl (C=O) on terminal carbon
26
New cards
Describe the possible structures of carbohydrates (sugars):
Usually linear but sometimes cyclic
27
New cards
Why doe 5 or more carbons form a ring structure?
Due to the reaction of a keto or aldehyde group with hydroxyl group
28
New cards
One of your family friend is complaining about his sleepless night because of the frequent urination, feeling tired and loosing weight. You remember that these symptoms look like diabetes. What do you think he needs to get it checked from the laboratory? Based on the initial carbohydrate information and their interaction what do you think you will ask him and what is the rationale behind to ask for a test you studied in your course?
He will need to get a HbA1c test done from the laboratory. This test will react the linear from of sugar with a protein molecule, if there is too much sugar in the blood the cyclic from of the molecule will not occur, rather it will react with the hemoglobin in the blood and will from a A1c haemoglobin molecule.
29
New cards
Explain how sugars react in diabetic patients:
The linear form of sugar with react with the hemoglobin protein (A1c) in stead of the cyclic form
30
New cards
Describe glycogen and give an example:
Branched animal product \~ alpha(1-4) strand with alpha(1-6) branch points, is the most branched, branched glucose polymers
31
New cards
Describe starch and give an example:
Branched plant product \~ made up of branched Amylopectin with unbranched amylose, mostly alpha(1-4), helical in structure
32
New cards
Describe cellulose and give an example:
Unbranched structural polysaccharide of plant product ~ unbranched glucose polymer beta(1-4), is linear
33
New cards
Describe the differences between alpha helices and beta sheets and give an example of each:
Alpha helices:
- right hand coil, rod like
- hydrogen bonds parallel to polypeptide backbone
- hydrogen bonds between NH group and C=O of amino acids
- only one type and exists in a single chain
- R groups of amino acids are outside of helix
- Ex. DNA, hemoglobin
Beta Sheets:
- sheet like
- hydrogen bonds are perpendicular to polypeptide backbone
hydrogen bonds between neighbouring NH and C=O of adjacent peptide chains
- can be parallel, antiparallel or both
- R groups of amino acids are both inside and outside of sheet
- Ex. Silk
- right hand coil, rod like
- hydrogen bonds parallel to polypeptide backbone
- hydrogen bonds between NH group and C=O of amino acids
- only one type and exists in a single chain
- R groups of amino acids are outside of helix
- Ex. DNA, hemoglobin
Beta Sheets:
- sheet like
- hydrogen bonds are perpendicular to polypeptide backbone
hydrogen bonds between neighbouring NH and C=O of adjacent peptide chains
- can be parallel, antiparallel or both
- R groups of amino acids are both inside and outside of sheet
- Ex. Silk
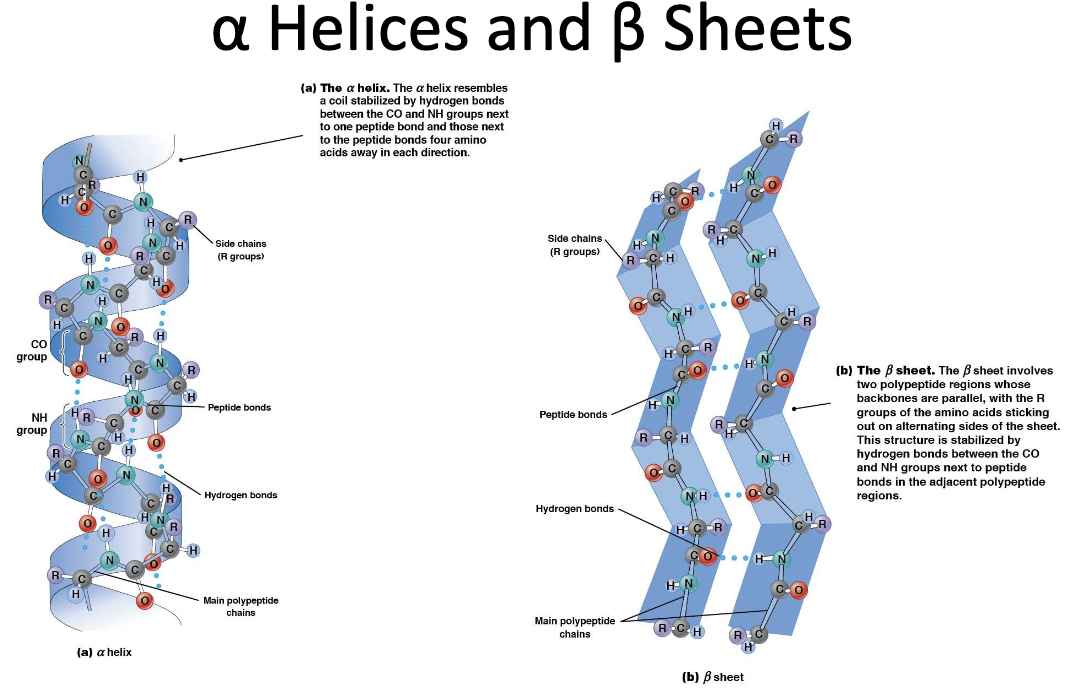
34
New cards
How can you denature proteins?
Using detergents (SDS), organic solvents, radiation, heat, change in pH, urea
35
New cards
Why would you want to denature a protein?
To study diseases
36
New cards
Explain Alzheimers disease:
Is a neurodegenerative disorder that results in dementia, disorientation, confusion, etc. Due to mis-folding of proteins.
Amyloid hypothesis: effect in gene plaque (beta-amyloid) formed which causes signal conduction to stop
Taupathies: Tau protein enters neuron and causes plaque buildup (beta-amyloid) which sops signal conduction
Amyloid hypothesis: effect in gene plaque (beta-amyloid) formed which causes signal conduction to stop
Taupathies: Tau protein enters neuron and causes plaque buildup (beta-amyloid) which sops signal conduction
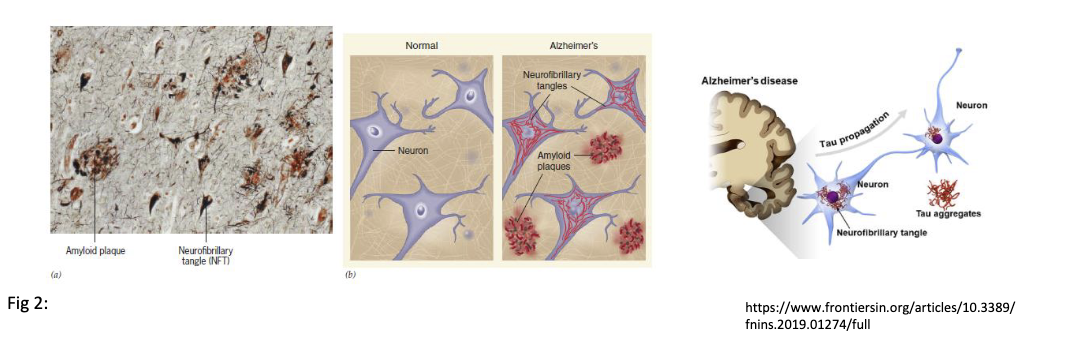
37
New cards
Using thermodynamics explain exothermic and endothermic reactions:
Loss of heat is exothermic. Gain of heat is endothermic.
38
New cards
What is delta G?
GIbbs free energy
39
New cards
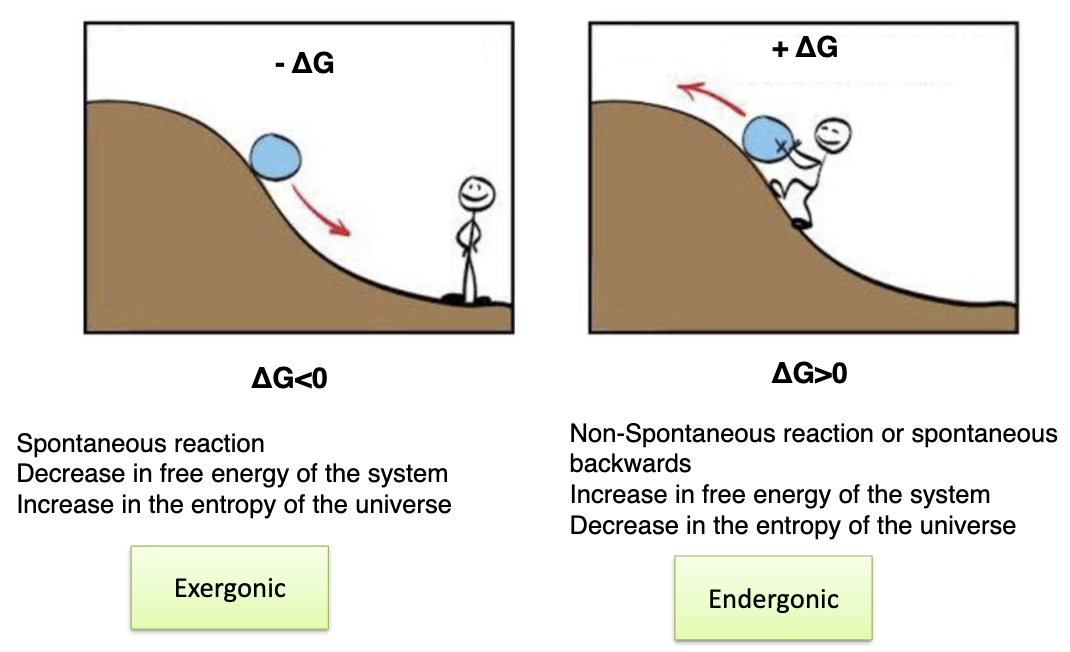
Explain spontaneous and non-spontaneous reactions:
+ve delta G is non-spontaneous and increase in free energy of system and decrease in entropy of universe
-ve delta G is spontaneous and decrease in free energy of system and increase un entropy of universe
-ve delta G is spontaneous and decrease in free energy of system and increase un entropy of universe
40
New cards
Why is ATP hydrolysis favourable? (4-5 mark question)
Entropy: decrease in free energy favours product formation which increases entropy
Electrostatic Repulsion: the adjacent negative charges of oxygen in ATP repel each other
Resonance stabilization of products: resonance stability of ADP and Pi is greater than ATP
Solvation of ADP, Pi and H+ id higher than ATP since ATP can lose its phosphate group easier than ADP can
Electrostatic Repulsion: the adjacent negative charges of oxygen in ATP repel each other
Resonance stabilization of products: resonance stability of ADP and Pi is greater than ATP
Solvation of ADP, Pi and H+ id higher than ATP since ATP can lose its phosphate group easier than ADP can
41
New cards
Characterize the properties of enzymes (3-5):
Enzymes are biological catalysts
- they are not prominently altered during a reaction
- they cannot effect the thermodynamics of a reaction (dont supply energy for reaction and doesnt determine reactants:product ration at equilibrium
- are highly specific
- they lower activation energy
- lower temperatures can lower activation energy and more molecules can react, this can also be applied to pH
- enzymes may not work in certain pH or temperatures
- present in cells in small amounts
42
New cards
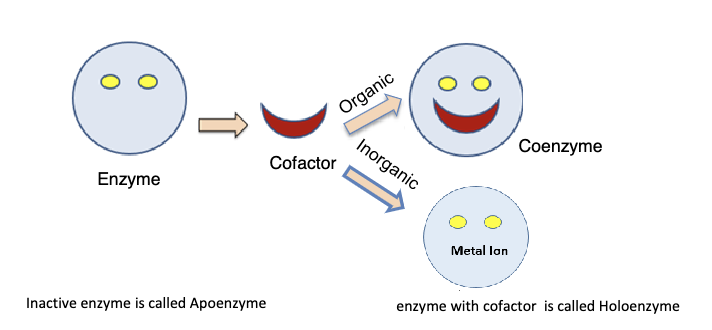
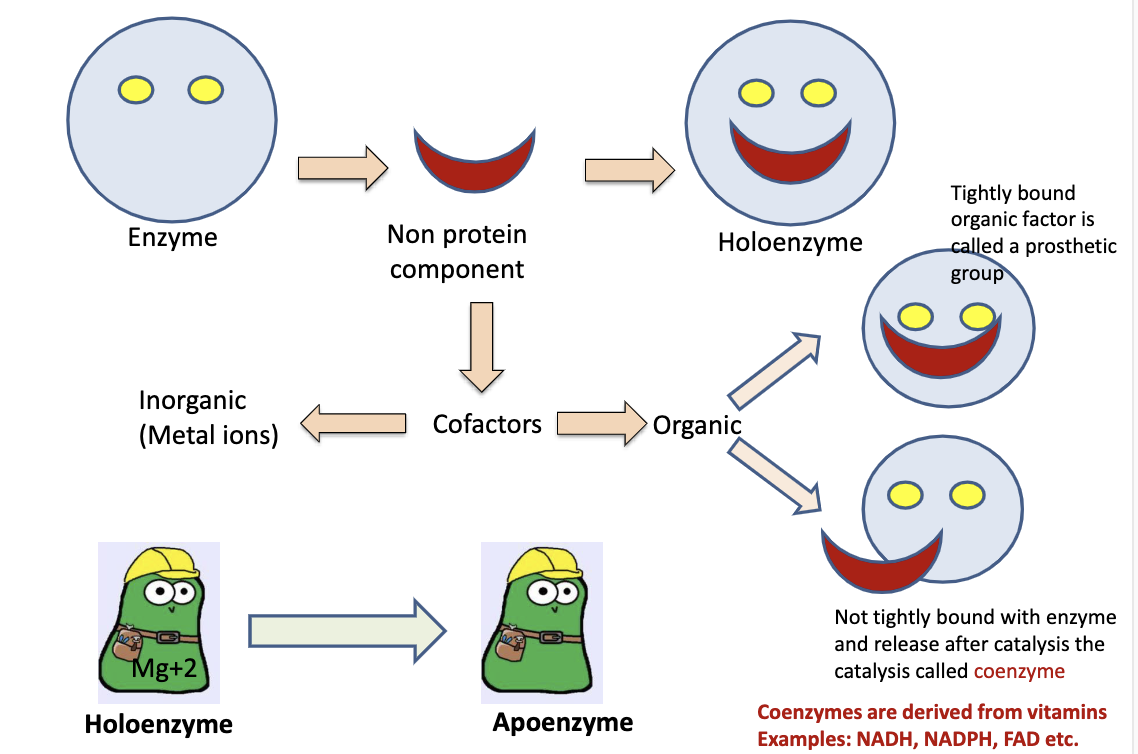
Explain significance of cofactors and coenzymes:
Both act as a "key" to start the reaction, will bind and activate an enzyme (inactive enzyme, no cofactor/coenzyme, is a apoenzyme). Cofactors are inorganic (metals), coenzymes are organic. Enzyme with cofactor is a holoenzyme
43
New cards
Describe the 2 active site models:
Lock and Key model: enzyme is stiff and substrate fits in perfectly, like a lock and key (hence the name)
Induced Fit model: the enzyme will change to fit the substrate
Enzyme-Substrate complex will bind using non-covalent forces
Substrates are specific to enzymes due to chemistry of active site
Induced Fit model: the enzyme will change to fit the substrate
Enzyme-Substrate complex will bind using non-covalent forces
Substrates are specific to enzymes due to chemistry of active site
44
New cards
What is a irreversible inhibitor (give an example)?
Is an inhibitor which binds tightly to enzyme through a covalent bond
Ex. nerve gas
Ex. nerve gas
45
New cards
What is a reversible inhibitor (give an example)?
inhibitor that binds loosely to enzyme, non-covalently. Two types competitive and non-completive
46
New cards
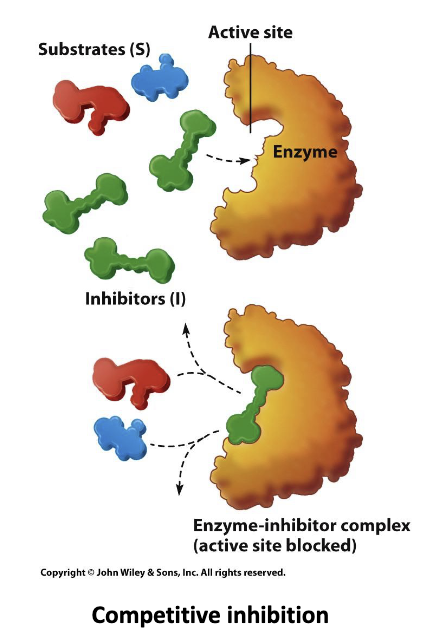
Describe competitive inhibitors:
Compete with substate for active site. Can be overcome if there is more substrate than inhibitor.
- Increases Km without affecting Vmax
Ex. ACE inhibitor that stops angiotensin II from binding to ACE and thus will lower blood pressure
- Increases Km without affecting Vmax
Ex. ACE inhibitor that stops angiotensin II from binding to ACE and thus will lower blood pressure
47
New cards
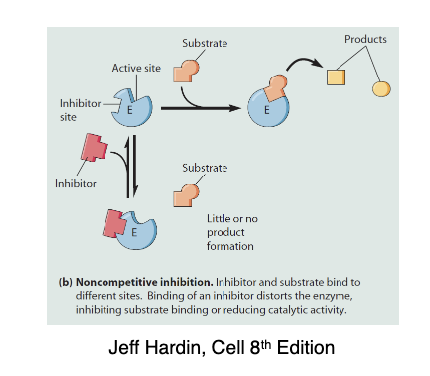
Descrie non-competitive inhibitors:
Does not bind to active site but instead binds to the allosteric site and will change the shape of the enzymes active site. Cannot be overcome with higher substrate to inhibitor ratio
* Decreases Vmax without affecting Km
* Ex. Tipranavir with inhibit the anti-retroviral protease inhibitor and is used for prevention and treatment of HIV
* Decreases Vmax without affecting Km
* Ex. Tipranavir with inhibit the anti-retroviral protease inhibitor and is used for prevention and treatment of HIV
48
New cards
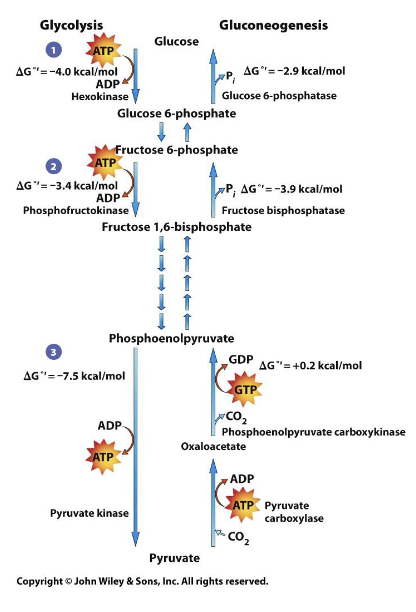
Which pathway is catabolic?
A) glycolysis
B) gluconeogenesis
A) glycolysis
B) gluconeogenesis
A) glycolysis since it is a BREAK down of glucose
- gluconeogenesis is the making of glucose from non carbohydrate sources
- gluconeogenesis is the making of glucose from non carbohydrate sources
49
New cards
What enzyme does phosphorylation?
(protein) kinases add a phosphate group to other proteins/molecules
50
New cards
What are the 3 types of membrane lipids
- Phospholipids
- Sphingolipids
- Cholesterol
- Sphingolipids
- Cholesterol
51
New cards
Describe a cerebroside:
Neutral glycolipid with an uncharged sugar a a head group.
Present in brain and nerve cells
Present in brain and nerve cells
52
New cards
Describe a ganglioside:
Has a oligosaccharide head group with 1 or more -ve charged sailic acid residues.
Present in brain and nerve cells
People who cannot synthesize this (G M3) suffer for a neurological disease that causes seizures and blindness
Present in brain and nerve cells
People who cannot synthesize this (G M3) suffer for a neurological disease that causes seizures and blindness
53
New cards
How are blood groups determined and why are they different?
They are based on the glycolipids since they have an enzyme that adds N-acetylgalactosamine to the ends of RBC membrane glycolipids. A lack of these proteins results in the O-blood group
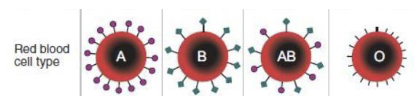
54
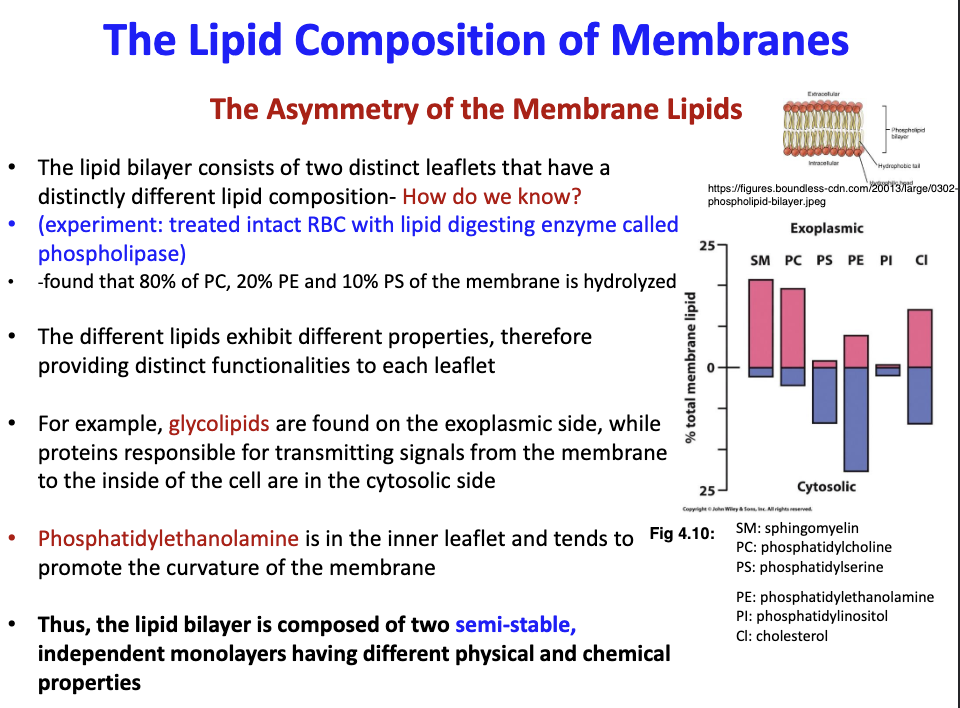
New cards
How did scientists figure out that the 2 leaflets of a membrane have different lipid compositions?
RBCs were treated with phospholipase. Since different lipids have different properties so each leaflet will have different properties. Responsible for the asymmetry of a membrane
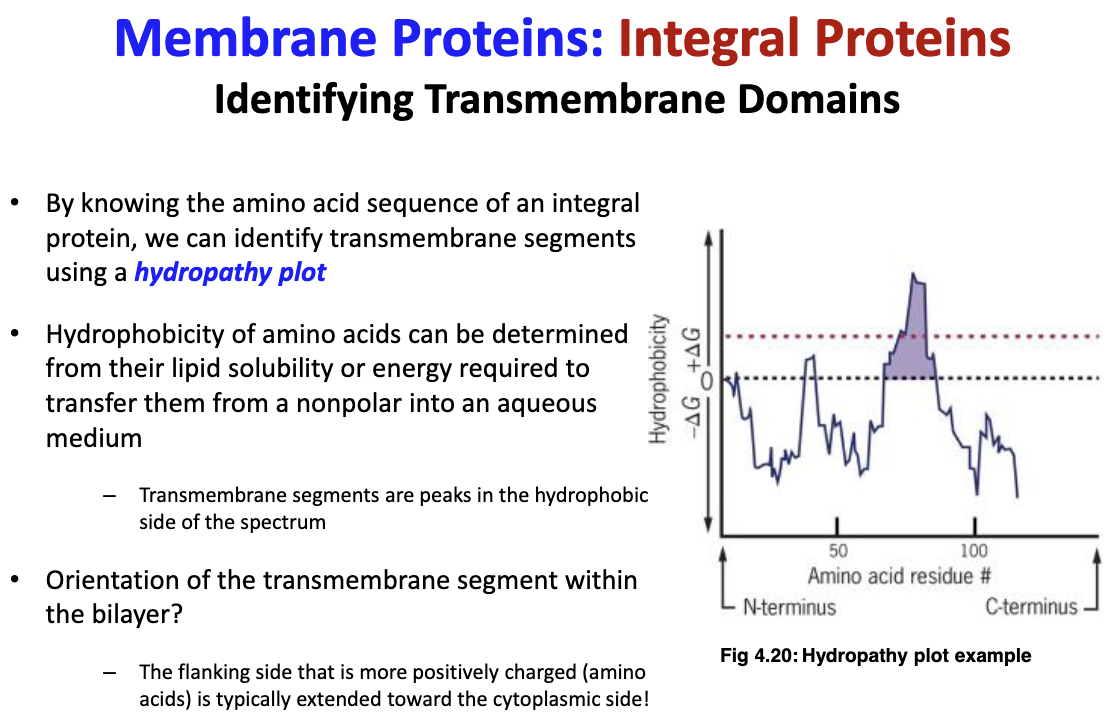
55
New cards
What is a hydropathy plot and what can it be used for?
can identify transmembrane segments of known amino acid sequences from integral proteins
56
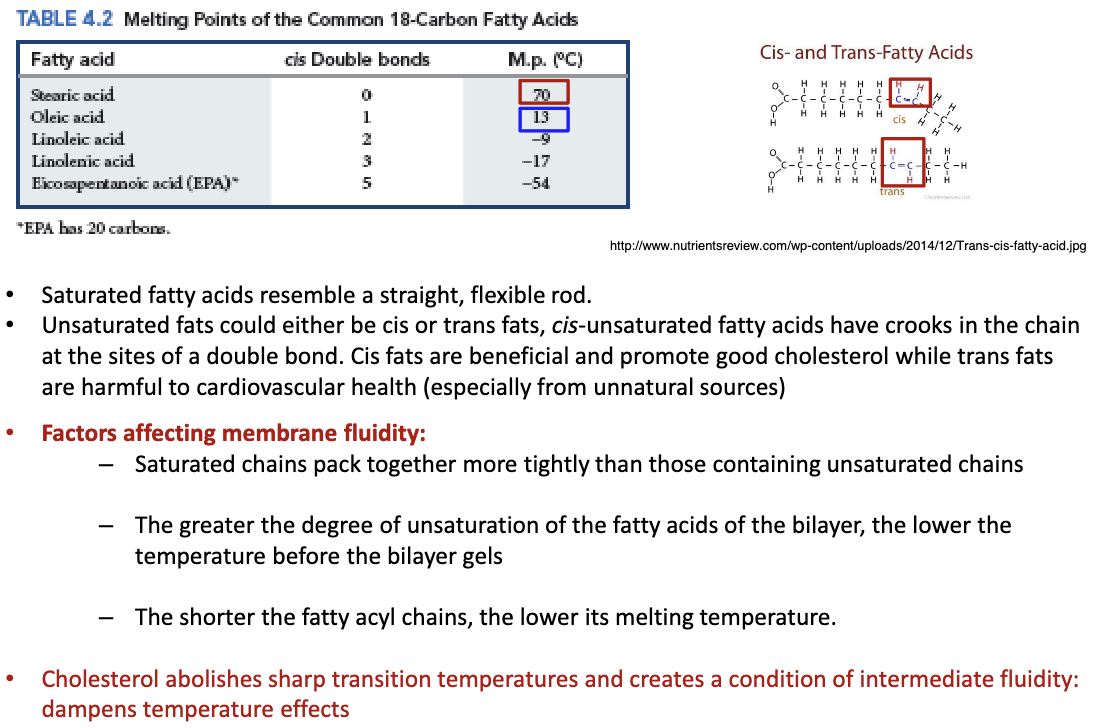
New cards
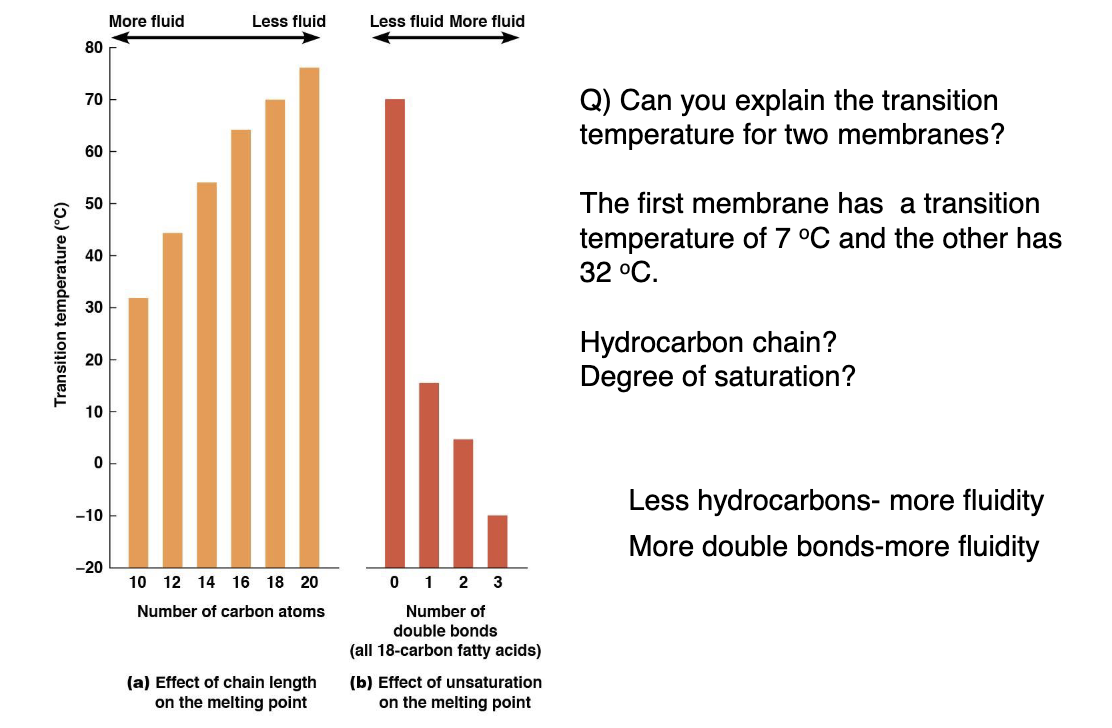
What factors effect membrane fluidity?
Higher temperatures make the membrane more fluid, lower temperatures does the opposite. The more unsaturated (shorter chain) the more fluid.
57
New cards
What happens when transition temperature of membrane is approached?
The lipid is more rigid as it it is converted into a frozen crystalline gel.
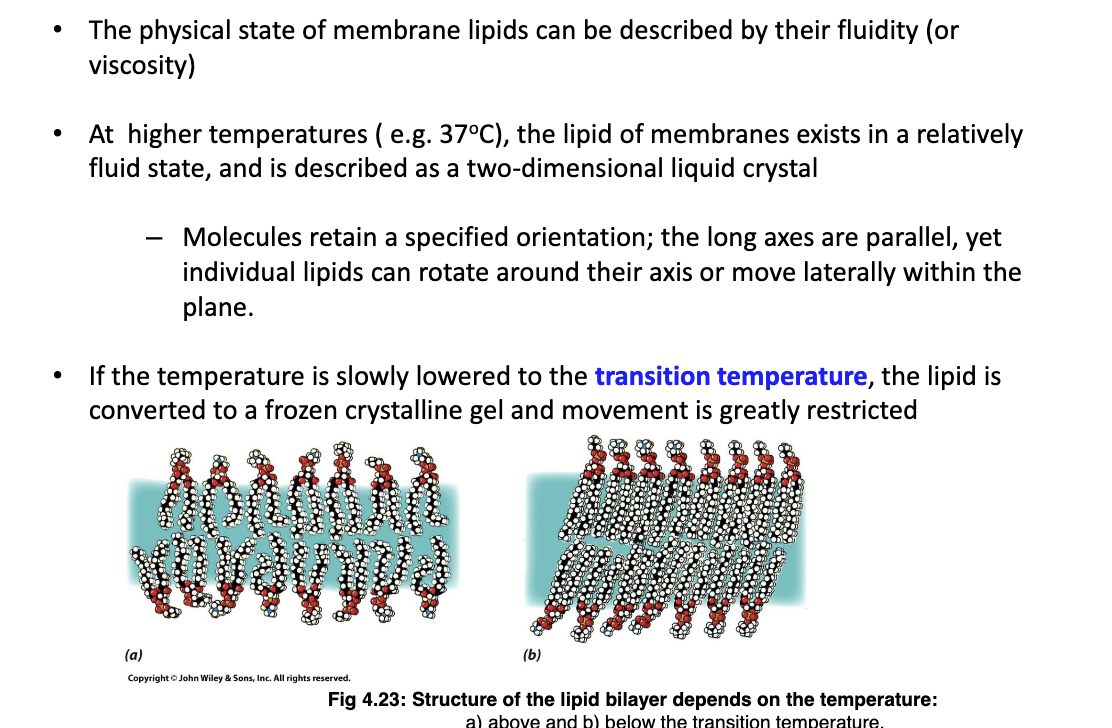
58
New cards
You got a project to analyze and prepare a report on the membrane fluidity. In the middle of the winter (13°C), the membranes from a tissue sample from an organism dwelling in a pond are analyzed for their phospholipid content. In the middle of the summer (29°C), another tissue sample is taken from the same type of organism and the membrane lipid content determined. The lipid content has changed. Please explain in your report how has it changed and why?
The lipid content of the sample taken in the summer will be higher than the lipid content of the sample taken in the winter. In the winter the membrane will be more unsaturated, making the membrane more fluid but due to the lipids crystallizing the membrane will be more rigid. In the winter the membrane will have more double bonds to make it fluid to counteract the effects of the cooler temperature. This would be the opposite in the summer.
59
New cards
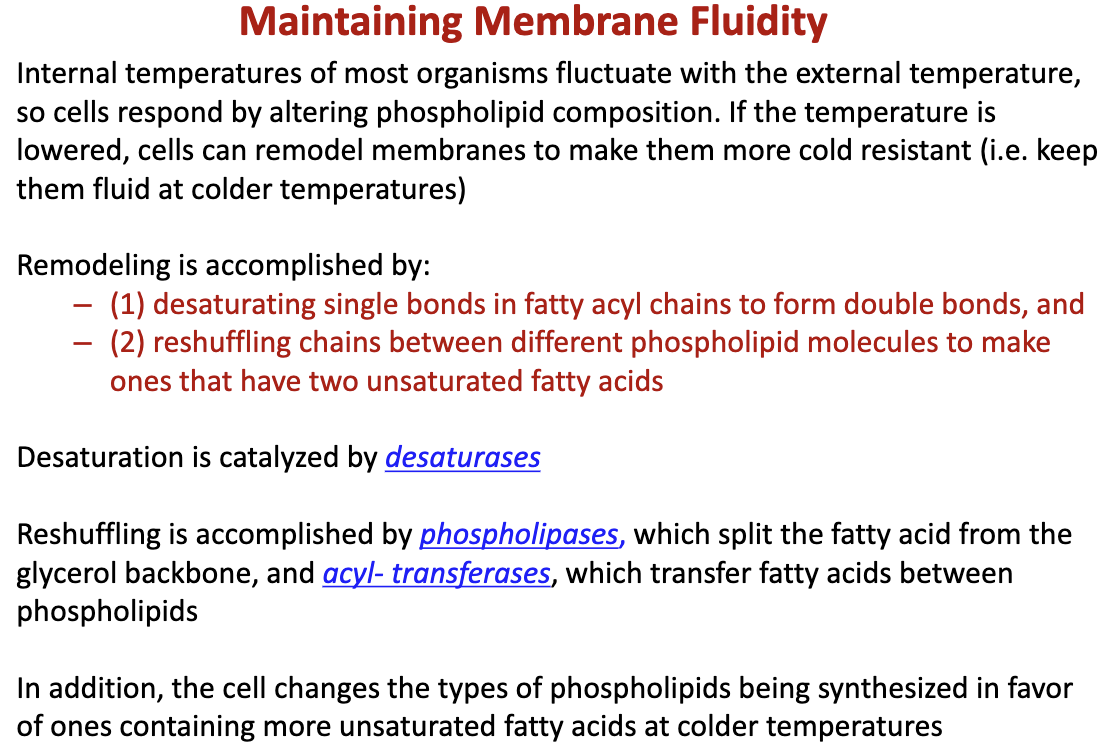
How do cells remodel themselves and why?
- Increasing the number of double bonds by desaturating single bonds, catalyzed by desaturases
- reshuffling of chains between different phospholipid molecules that to make them have 2 unsaturated fatty acids
- reshuffling of chains between different phospholipid molecules that to make them have 2 unsaturated fatty acids
60
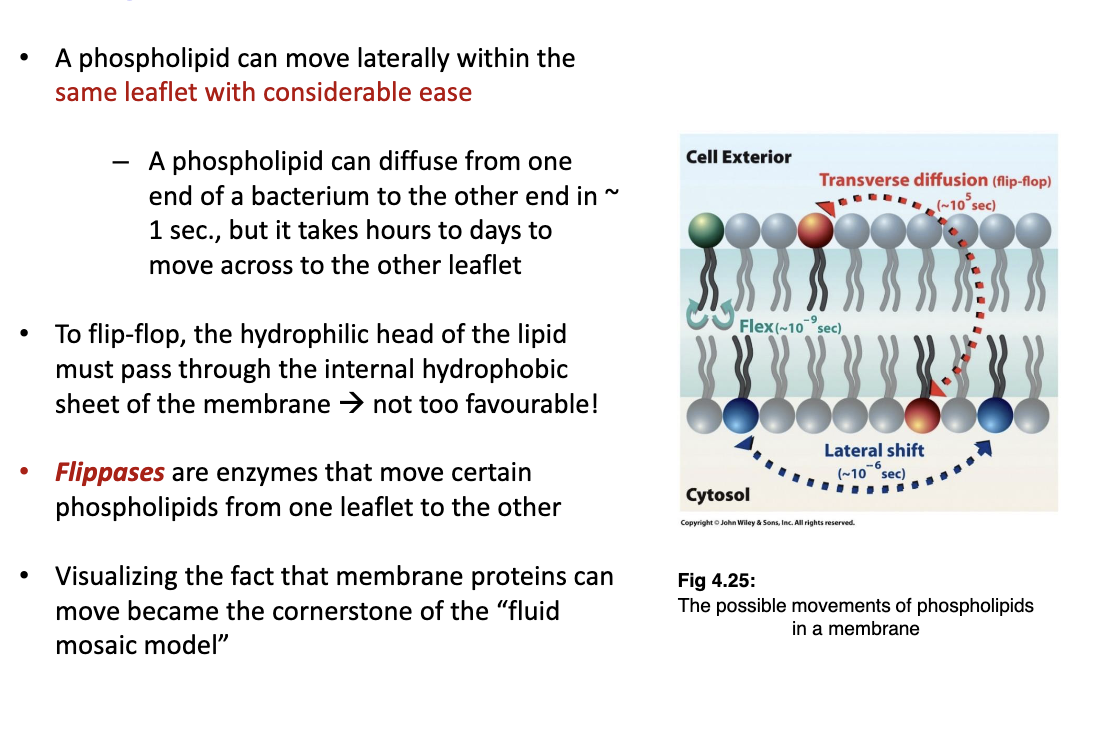
New cards
How are phospholipids flipped between leaflets?
Through the flipase enzyme
61
New cards
What are 5 types of diffusion?
1. simple diffusion through bilayer
2. simple diffusion through aqueous channel
3. ion diffusion channels
4. facilitated diffusion
5. active transpost
2. simple diffusion through aqueous channel
3. ion diffusion channels
4. facilitated diffusion
5. active transpost
62
New cards
What does simple diffusion rely on?
Relies on molecular size and polarity. Smaller molecules penetrates the lipid bi-layer faster than larger ones, polar molecules (sugars and amino acids) poorly penetrate membranes, greater the lipid solubility the greater the penetration
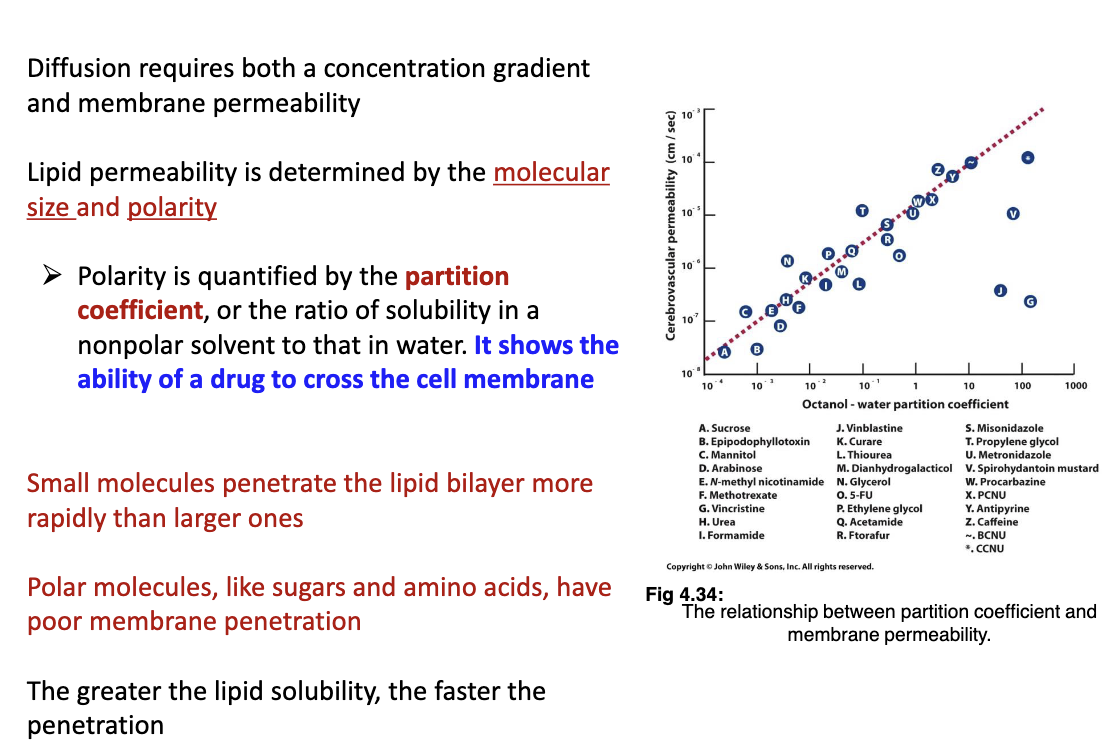
63
New cards
Describe active transport:
ATP is needed for this transport to occur.
Ex. the sodium-potassium pump (ATPase) needs ATP to function and let in 2 potassium and let out 3 sodium. The ATP is phosphorylated/hydrolyzed for the diffusion to occur
Ex. the sodium-potassium pump (ATPase) needs ATP to function and let in 2 potassium and let out 3 sodium. The ATP is phosphorylated/hydrolyzed for the diffusion to occur
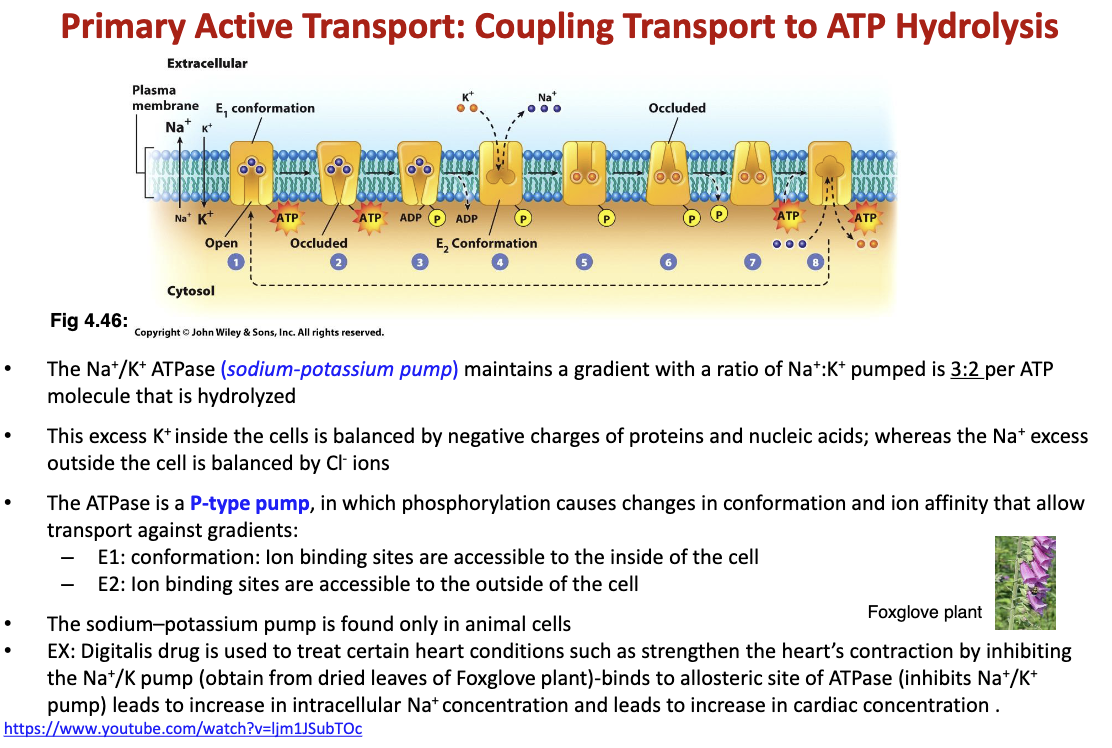
64
New cards
What are characteristics of neurotransmitters?
They are chemicals created and released from the presynaptic cleft and diffuses into the postsynaptic cleft
65
New cards
Can transmitters excite or inhibit the postsynaptic cell?
They cab depolarize (excite) or hyper-polarize (inhibit). They bind to ion channel receptors and simulates/inhibits function
66
New cards
How are neurotransmitters maintained in our body?
Their action is terminated by re-uptake or enzymatic breakdown
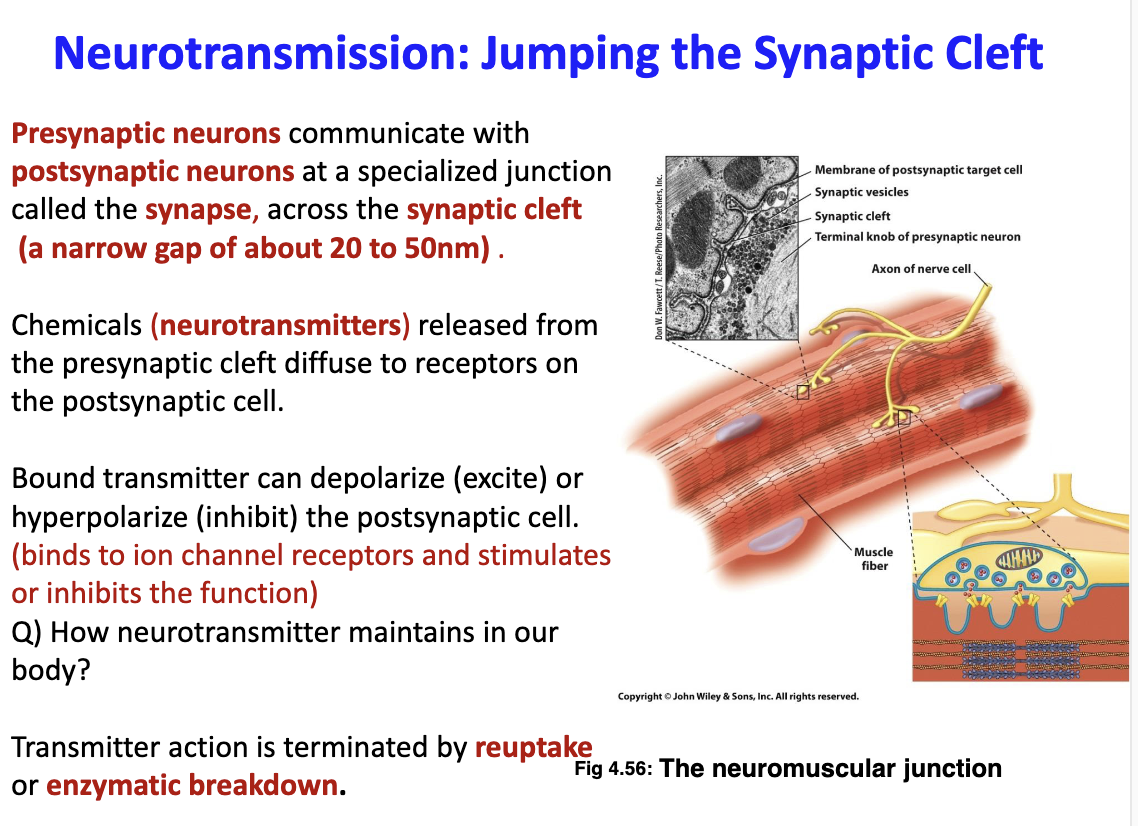
67
New cards
What is the function of procaine and novocaine?
They act as sodium channel blockers by blocking the ion channels in the membrane of sensory cells and neurons. This results is the inhibition of the action potential of the affected cells (skin or teeth) and the brain does not receive info about the stimulus.
68
New cards
What is the uses of eliminating neurotransmitters?
Neurotransmitters can be eliminated through enzymes that will destroy the neurotransmitters in synaptic cleft and the neurotransmitter re-uptake process.
Ex. cocaine inhibits the re-uptake of dopamine in the synaptic cleft which causes a short feeling of euphoria and makes one want to repeate the activity
Ex. cocaine inhibits the re-uptake of dopamine in the synaptic cleft which causes a short feeling of euphoria and makes one want to repeate the activity
69
New cards
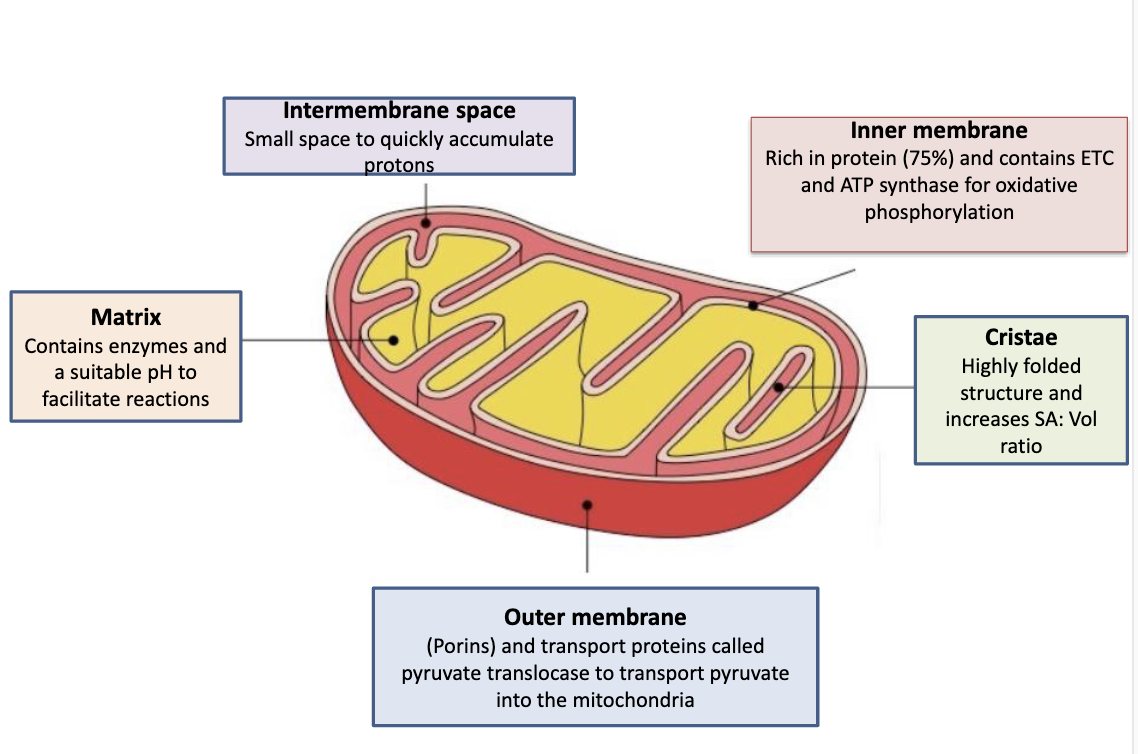
Where is the cristae and cisternae in a cell?
The cristae is the inner boundary membrane of the mitochondria. The cisternae is a network of flattened sacks found in the RER and golgi
70
New cards
Where does glycolysis occur?
Cytoplasm of a cell
71
New cards
Where does the TCA (krebs cycle or citric acid cycle)
Matrix of mitochondria
72
New cards
Where does the ETC (electron transport chain) occur?
Inner mitochondrial membrane
73
New cards
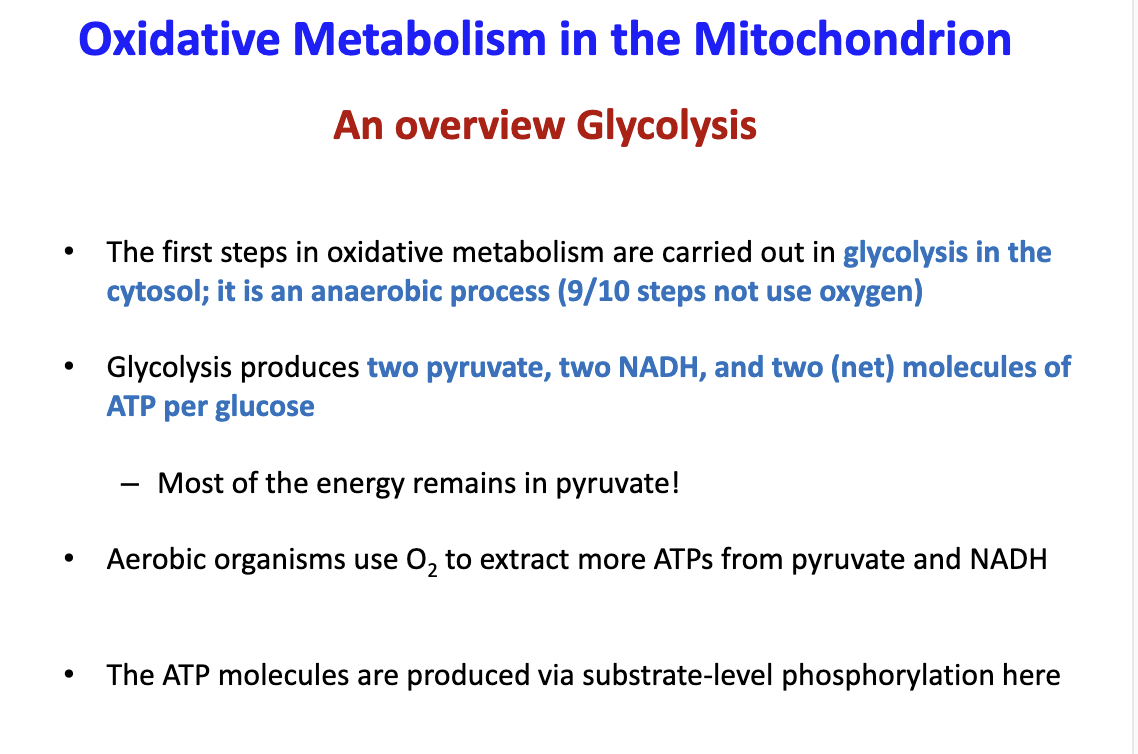
Give a brief overview of glycolysis?
First step occurs in the cytoplasm and doesnt use oxygen (anaerobic). Glycolysis will produce 2 pyruvate, 2 NADH and net 2 ATP per glucose. Most of the energy is in pyruvate. ATP is produced by substrate-level phosphorylation
74
New cards
How many total carbons form 1 glucose molecule will enter the TCA cycle?
4
75
New cards
How many total carbons from the original glucose molecule will enter the TCA cycle without oxygen present?
0
76
New cards
What is the difference between a holoenzyme and a apoenzyme?
Holoenzyme has a cofactor while an apoenzyme does not
77
New cards
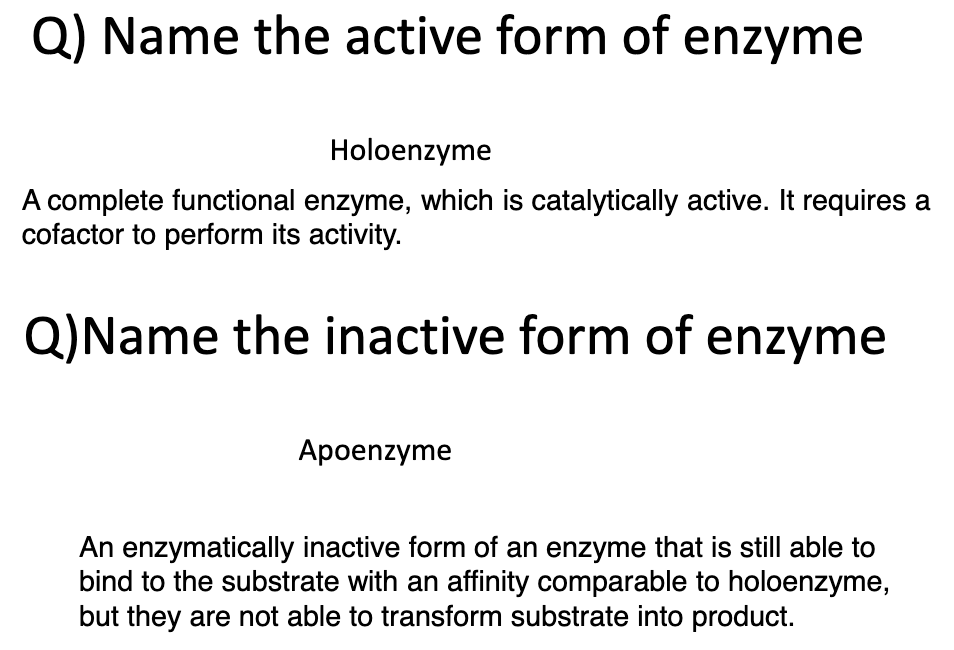
Active form of an enzyme is:
holoenzyme
78
New cards
Inactive form of an enzyme is:
apoenzyme
79
New cards
What are the 5 types of electron carriers found the membrane of a mitochondria?
1. Flavoproteins
2. Cytochromes
3. Copper atoms
4. Ubiquinone
5. Iron-sulphur proteins
80
New cards
What are flavoproteins?
Are polypeptides bound to FAD and FMN which can donate 2p+ and 2e-. Major flavoproteins are NADH dehydrogenase of the ETC and succinate dehydrogenase of the TCA. They are found in the mitochondria
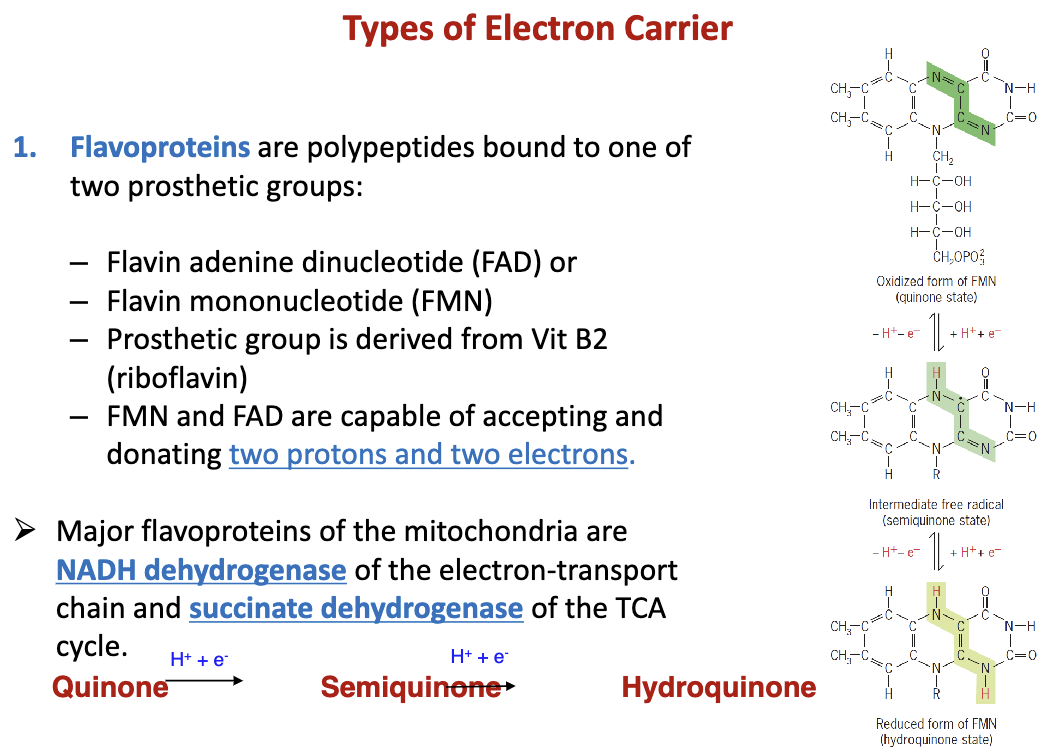
81
New cards
What are cytochromes?
Contain heme prosthetic groups having Fe or Cu ions. Found in the cristae of mitochondria. Cytochrome c is a soluble protein found in the inter-membrane space and is apart of the ETC.
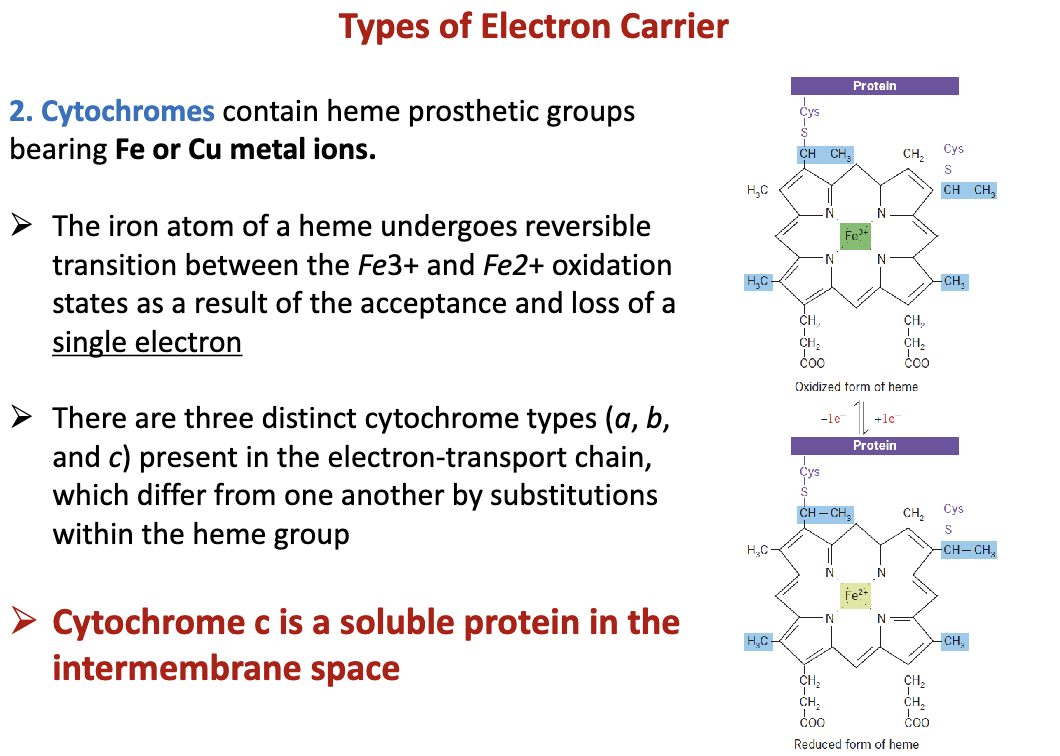
82
New cards
What is ubiquinone?
Also known coenzyme Q which is lipid soluble. Able to accept 2e- and 2p+
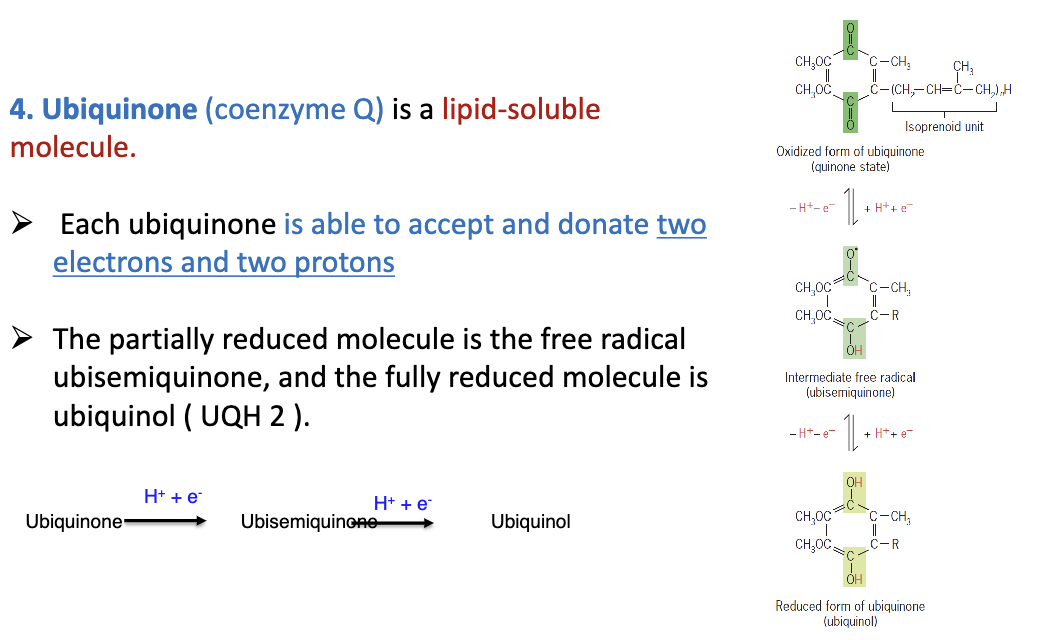
83
New cards
What are the Electron-Transport Complexes?
Complex I: NADH dehydrogenase, is a catalyst for the transfer of e- from NADH to ubiquinone and transports 4H+ per pair of e-
Complex II: succinate dehydrogenase, catalyst for transfer of e- from succinate to FAD to ubiquinone without H+, used in both TCA and ETC
Complex III: cytochrome bc1, catalyst for the transfer of e- from ubiquinone to cytochrome c and transports 4H+ per pair of e-
Complex IV: cytochrome c oxidase, catalyst for transfer of e- to O2 and transports 2H+ per pair of e- across inner membranes
Complex II: succinate dehydrogenase, catalyst for transfer of e- from succinate to FAD to ubiquinone without H+, used in both TCA and ETC
Complex III: cytochrome bc1, catalyst for the transfer of e- from ubiquinone to cytochrome c and transports 4H+ per pair of e-
Complex IV: cytochrome c oxidase, catalyst for transfer of e- to O2 and transports 2H+ per pair of e- across inner membranes
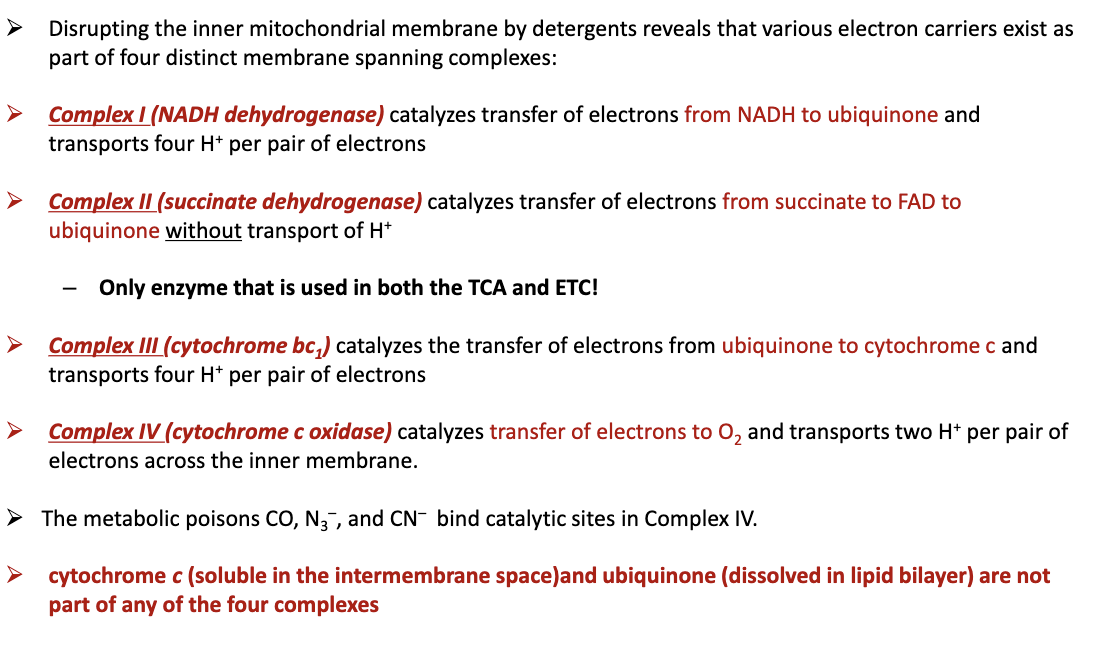
84
New cards
Are cytochrome c and ubiquinone apart of the 4 complexes?
no
85
New cards
Expand more on Complex I:
Carries out electron transfer and proton translocation
NADH --> FMN --> 7Fe-S complexes --> UQ
Dysfunction is linked to neurological diseases
NADH --> FMN --> 7Fe-S complexes --> UQ
Dysfunction is linked to neurological diseases
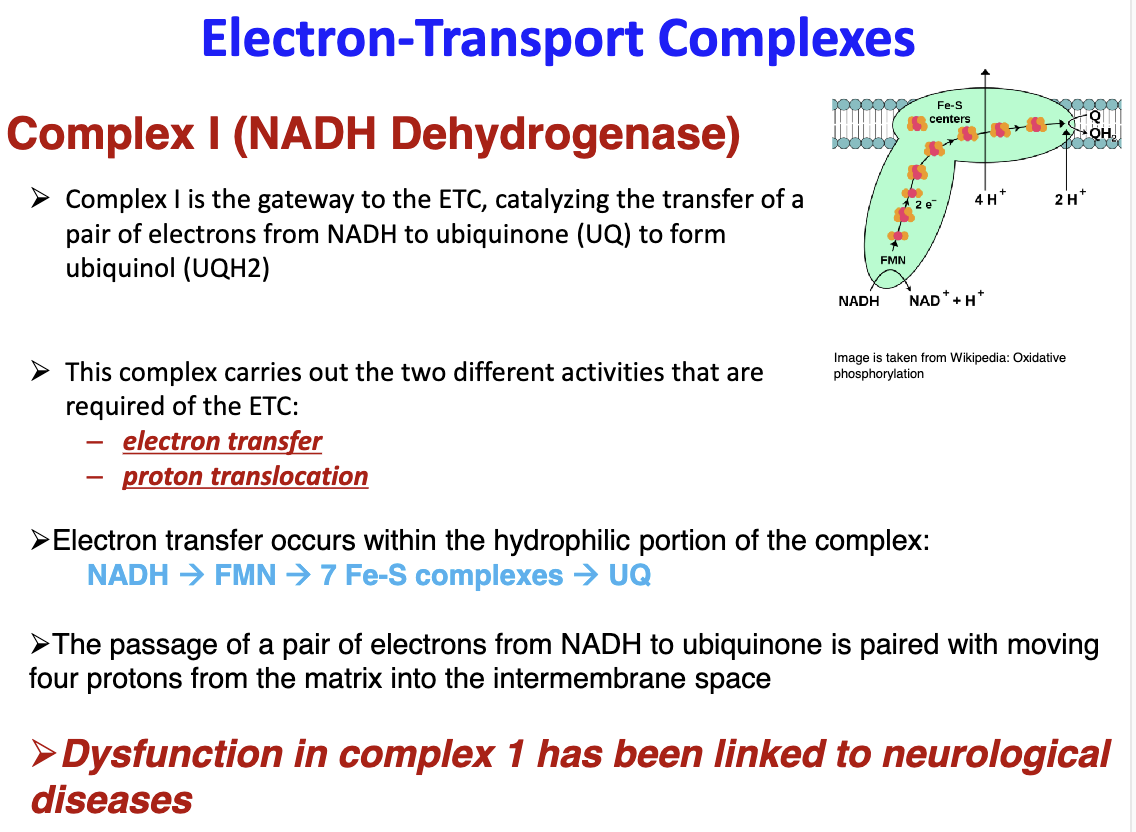
86
New cards
Explain Proton-Motive Force:
Background: deltaP is pH gradient which is the conc. gradient of H+ ions between matrix and inter-membrane space; the separation of charge across the membrane created a electric potential (trident symbol); are components of proton gradient
The energy present in both components is the proton-motive force (deltaP)
The energy present in both components is the proton-motive force (deltaP)
87
New cards
What is the significance of protein uncoupling?
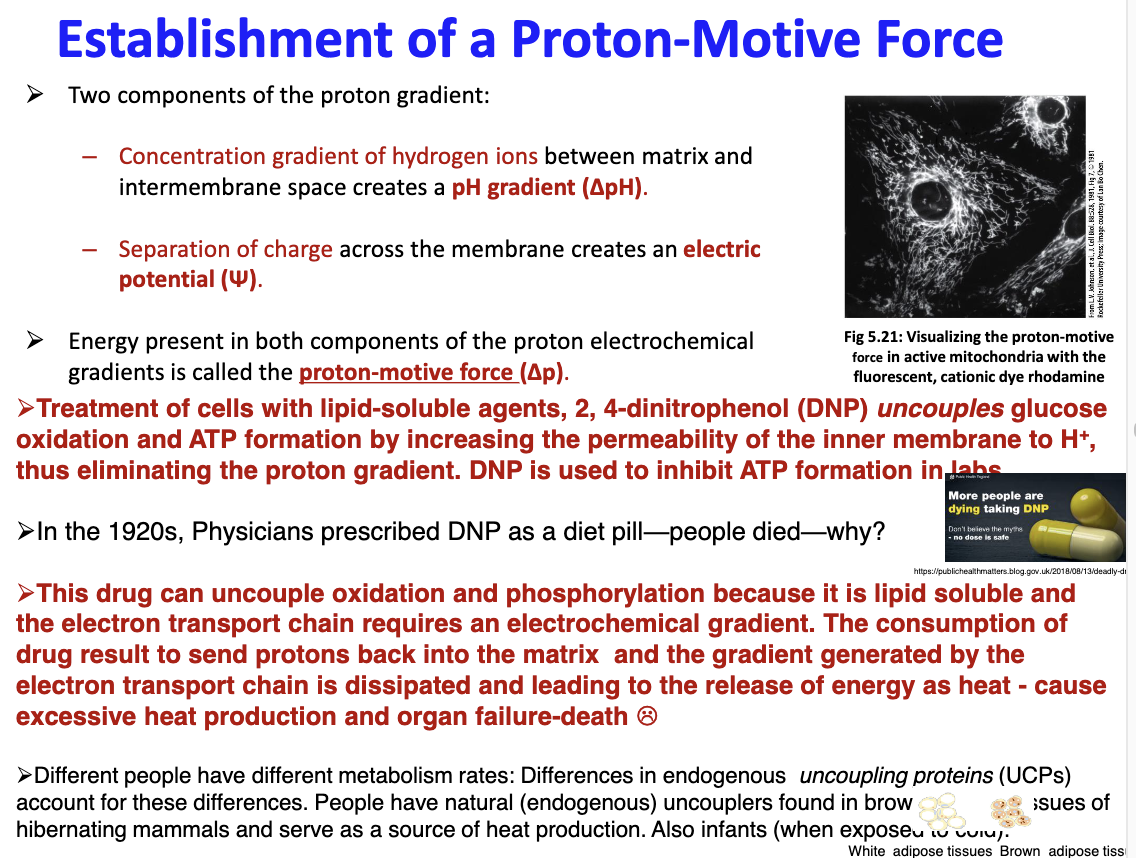
88
New cards
A patient came to the emergency room in the Toronto Western Hospital. He told doctor tat he is trying to reduce weight and very successful in losing weight rapidly in the last couple of days, but complains that he has lost energy and is tired most of the time. During his interview with the patient, the doctor thinks he has discovered what is causing the man's condition. What do you think he has found?
The doctor found that the patient was using uncoupling proteins to his advantage in weight loss, but due to protein uncoupling drugs such as DNP causing electrons to be sent back into the matrix which dissipates the gradient created by the electron transport chain which releases energy; this can account for the loss of energy in the patient.
89
New cards
What is the structure of ATP synthase?
2 parts; F1 and F0
F1 is the catalytic subunit and has three catalytic sites (3 alpha, 3 beta and 1 gamma) for ATP synthase, has 5 subunits ~ 3 alpha, 2 beta, 1 delta, 1 epsilon and 1 gamma
F0 attaches to F1 and contains a channel that allows p+ to be conduced from inter-membrane space to matrix, has 3 subunits ~ 1a, 2b and 10-14c
F1 is the catalytic subunit and has three catalytic sites (3 alpha, 3 beta and 1 gamma) for ATP synthase, has 5 subunits ~ 3 alpha, 2 beta, 1 delta, 1 epsilon and 1 gamma
F0 attaches to F1 and contains a channel that allows p+ to be conduced from inter-membrane space to matrix, has 3 subunits ~ 1a, 2b and 10-14c
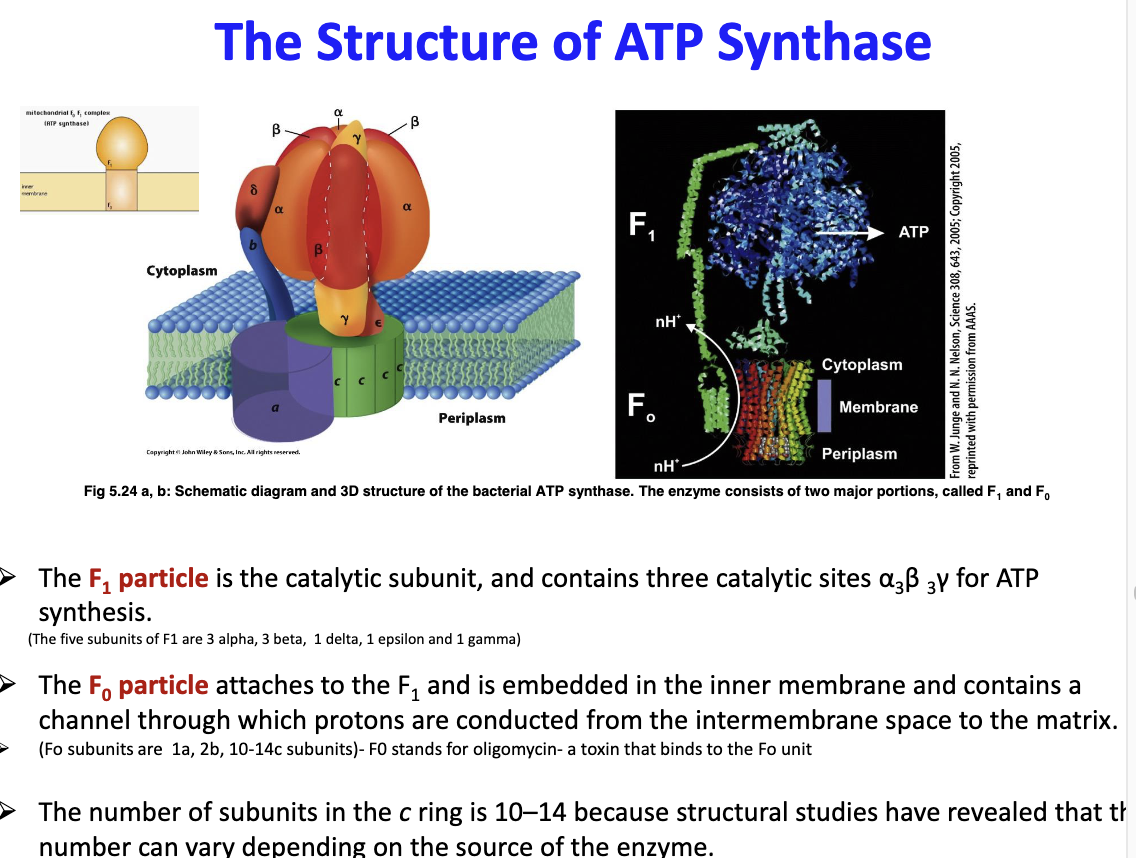
90
New cards
What is chemiosmosis?
The coupling of H+ translocation to ATP synthase
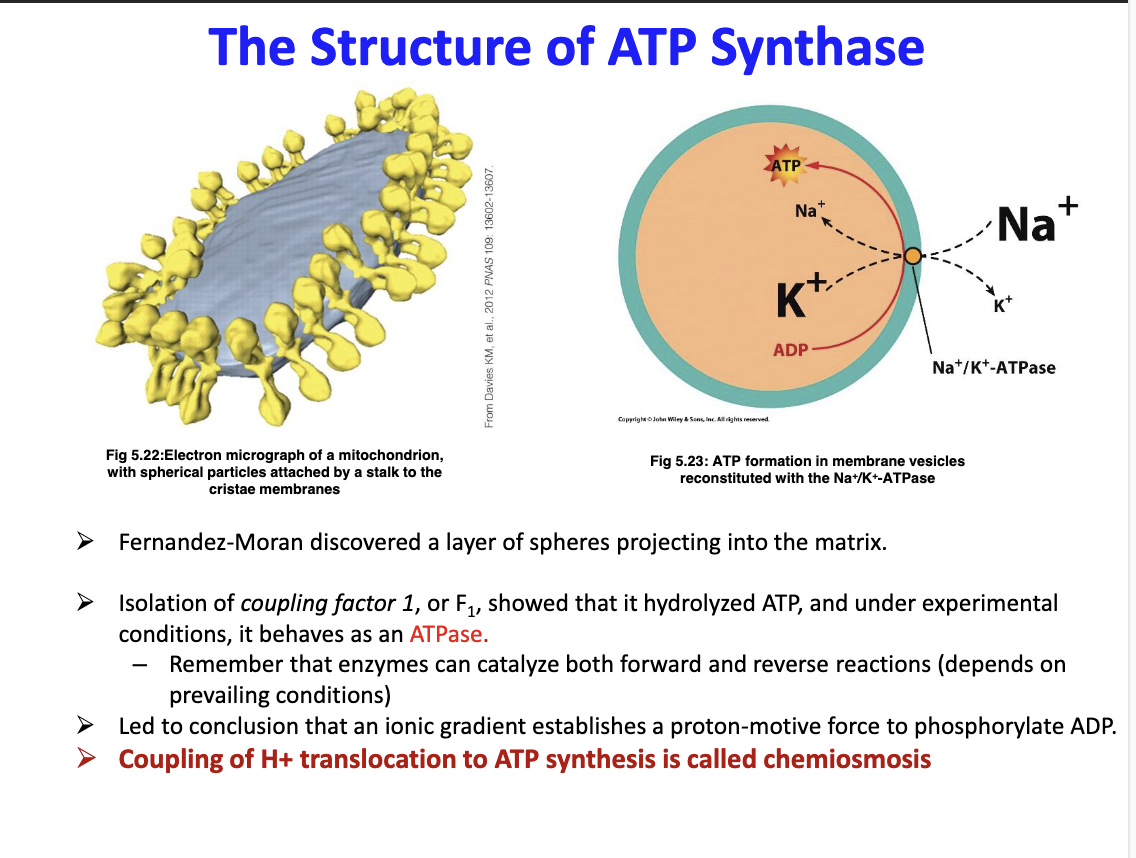
91
New cards
Explain peroxisomes:
Are oxidative organelles which breakdown toxic material in cells through the presence of at least 50 digestive and oxidative enzymes. They also contain catalase (decomposes hydrogen peroxide). causes Zellweger syndrome (ZS) wich results in neurological, visual and liver issues that lead to death in infants
92
New cards
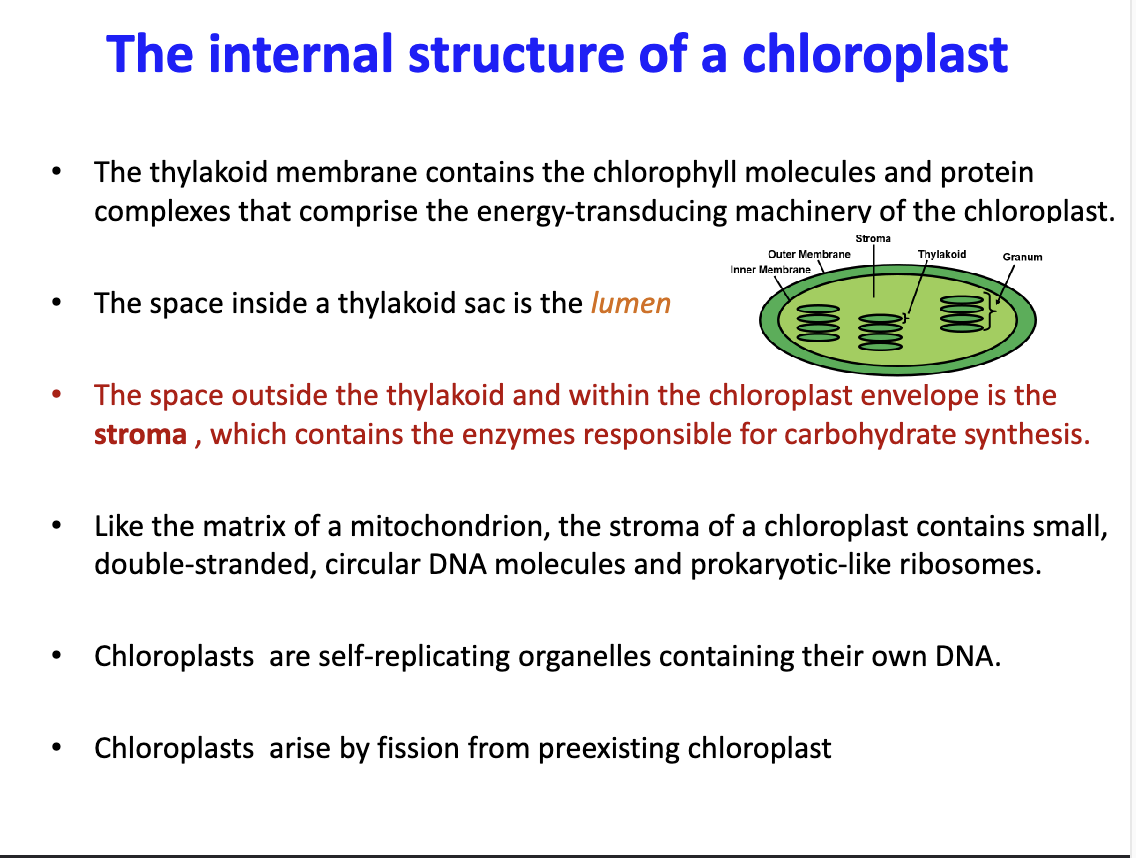
What does the stroma of a chloroplast contain?
Contains thylakoids in stacks known as granum. Also contains enzymes responsible for carbohydrate synthesis. Also contains small double stranded DNA and ribosomes
93
New cards
What ion is important for chlorophyll molecules?
Mg+ ion is central to chlorophyll molecules
94
New cards
What is the difference between light and dark dependant reactions?
Light dependant: sunlight is absorbed that converts ATP into NADPH
Light independent: uses energy in ATP and NADPH to make carbohydrates
Light independent: uses energy in ATP and NADPH to make carbohydrates
95
New cards
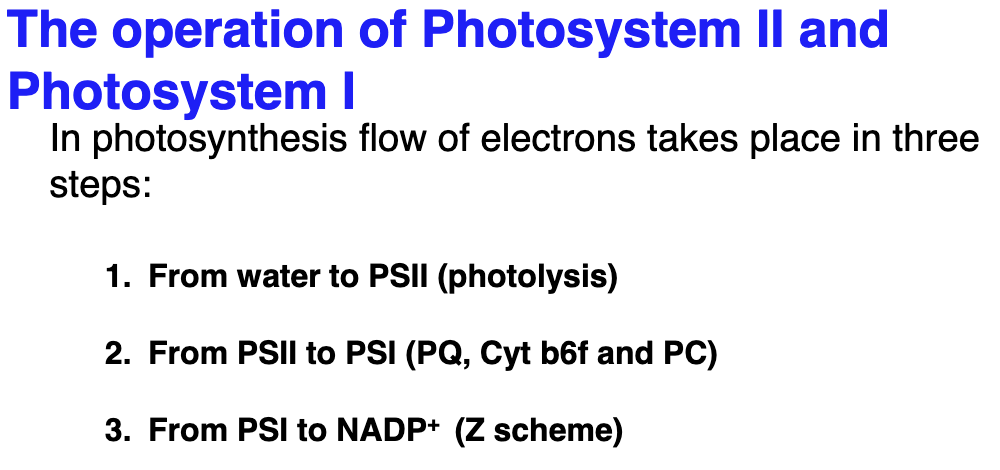
In basic terms explain the operation of PSI and PSII:
An electron from H2O enters PSII which boosts e- from below energy level of H2O then it moves to PSI which boosts the e- to a level above NADP+. This process of flow of e- from H2O to NADPH+ is called the Z scheme
96
New cards
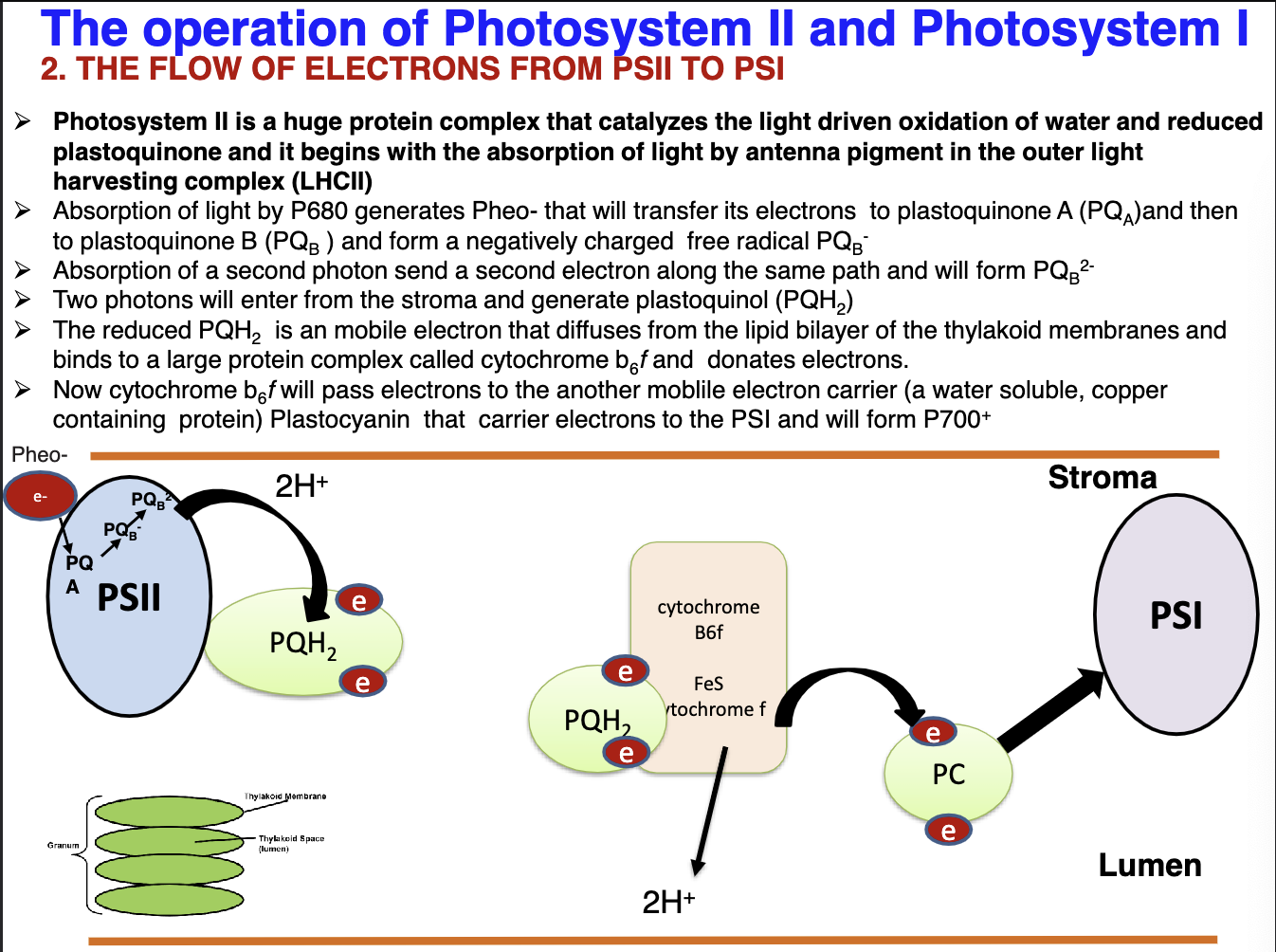
Explain the flow of e- from PSII (P680) to PSI (P700):
PSII is responsible for the catalyzation of the light driven process that reduces water and reduction of plastoquinone(PQ). The process starts when light is absorbed by the outer antenna pigment LHCII. Then Pheo- is generated which will transfer e- to PQA and then to PQB to for the negatively charged radical PQB-. The second photon is absorbed and a second electron will be sent through the path mentioned above creating PQB2-. After this photons will enter from the stroma and make plastoquinol (PQH2). Then this complex will diffuse through the thylakoid membrane to bind to cytochrome b6f and will donate its e-. This complex will donate its e- to plastocyanin that will carry e- to PSI to make P700+
97
New cards
Explain the flow of e- from PSI (P700) to NADP:
Light is absorbed by LHCI and passed to P700. This leads to the making of P700- and chlorophyll a (A0-). Then the NADP+ is catalyzed by the enzyme Ferreodoxin-NADP+ reductase, which contains a FAD prosthetic group and accepts 2e-
98
New cards
How is the positive charge of P700 pigment neutralized?
Plastocyanin will carry e- to the luminal side of PSI and then transfer these e- to P700+
99
New cards
What is the main function of PSI?
To reduce NADP to NADPH
100
New cards
How many photons are absorbed for the photosynthetic reaction to occur and what is the reaction that occurs?
There are 8 photons absorbed and the following reaction occurs:
2H2O + 2NADP+ --> O2 + 2NADPH
2H2O + 2NADP+ --> O2 + 2NADPH